Loteprednol Etabonate for the Treatment of Dry Eye Disease
- PMID: 32391735
- PMCID: PMC7482125
- DOI: 10.1089/jop.2020.0014
Loteprednol Etabonate for the Treatment of Dry Eye Disease
Abstract
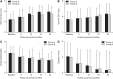
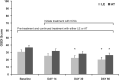
Dry eye disease (DED) is a common ocular condition that can impair vision and may adversely impact quality of life. Due to the inflammatory nature of this disorder, topical corticosteroids are an effective treatment option, particularly for moderate-to-severe DED when first-line treatments, such as ocular lubricants, are insufficient. Loteprednol etabonate (LE) is a retrometabolically designed corticosteroid with a low propensity to cause corticosteroid-related adverse effects, such as elevated intraocular pressure (IOP). This review was conducted to provide an assessment of published studies on the use of LE for treatment of inflammation associated with DED. Twelve prospective and 2 retrospective studies evaluating LE ophthalmic suspension 0.5% and 2 prospective studies evaluating LE ophthalmic gel 0.5% were identified. LE given as monotherapy or with artificial tears (AT) improved signs of DED, especially among patients with a more pronounced inflammatory component, and also improved DED symptoms compared to baseline and/or control. Treatment with LE before cyclosporine A (CsA) therapy reduced stinging upon CsA initiation and provided more rapid relief of DED signs and symptoms than CsA plus AT alone. In patients with meibomian gland dysfunction, LE alone, or in addition to eyelid scrubs/warm compresses, reduced clinical signs and symptoms, and tear proinflammatory cytokine levels. Overall, LE was safe and well tolerated, with minimal effects on IOP. While larger and longer-term studies are warranted, these data support the use of LE as a safe and effective treatment option for DED.
Keywords: dry eye disease; inflammation; intraocular pressure; loteprednol etabonate; ocular surface.
Conflict of interest statement
K.B. is a consultant for Alcon, Allergan, Bausch + Lomb, a division of Bausch Health US, LLC, Eyevance Pharmaceuticals, Kala Pharmaceuticals, Novartis, and Sun Pharmaceutical Industries Ltd. J.K. is a consultant for Allergan, EyePoint Pharmaceuticals, Inc., Novartis, and Ocular Science; he served on an ad board with Eyevance Pharmaceuticals, Kala Pharmaceuticals, and Ocular Therapeutix. P.M. is a consultant for Alcon, Allergan, Bausch + Lomb, a division of Bausch Health US, LLC, Bio-Tissue, Inc., Dompé, Eyevance Pharmaceuticals, Kala Pharmaceuticals, Novaliq, Novartis, and Sun Pharmaceutical Industries Ltd. A.R. is a consultant for Alcon, Allergan, Bausch + Lomb, a division of Bausch Health US, LLC, Kala Pharmaceuticals, Novartis, and Sun Pharmaceutical Industries Ltd.
Figures
References
-
- Craig J.P., Nichols K.K., Akpek E.K., et al. . TFOS DEWS II definition and classification report. Ocul. Surf. 15:276–283, 2017 - PubMed
-
- Bron A.J., de Paiva C.S., Chauhan S.K., et al. . TFOS DEWS II pathophysiology report. Ocul. Surf. 15:438–510, 2017 - PubMed
-
- Stapleton F., Alves M., Bunya V.Y., et al. . TFOS DEWS II epidemiology report. Ocul. Surf. 15:334–365, 2017 - PubMed
-
- Farrand K.F., Fridman M., Stillman I.Ö., and Schaumberg D.A.. Prevalence of diagnosed dry eye disease in the United States among adults aged 18 years and older. Am. J. Ophthalmol. 182:90–98, 2017 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
