Diagnostic Performance of RO948 F 18 Tau Positron Emission Tomography in the Differentiation of Alzheimer Disease From Other Neurodegenerative Disorders
- PMID: 32391858
- PMCID: PMC7215644
- DOI: 10.1001/jamaneurol.2020.0989
Diagnostic Performance of RO948 F 18 Tau Positron Emission Tomography in the Differentiation of Alzheimer Disease From Other Neurodegenerative Disorders
Abstract
Importance: The diagnostic performance of second-generation tau positron emission tomographic (PET) tracers is not yet known.
Objective: To examine the novel tau PET tracer RO948 F 18 ([18F]RO948) performance in discriminating Alzheimer disease (AD) from non-AD neurodegenerative disorders.
Design, setting, and participants: In this diagnostic study, 613 participants in the Swedish BioFINDER-2 study were consecutively enrolled in a prospective cross-sectional study from September 4, 2017, to August 28, 2019. Participants included 257 cognitively unimpaired controls, 154 patients with mild cognitive impairment, 100 patients with AD dementia, and 102 with non-AD neurodegenerative disorders. Evaluation included a comparison of tau PET tracer [18F]RO948 with magnetic resonance imaging (MRI) and cerebrospinal fluid and a head-to-head comparison between [18F]RO948 and flortaucipir F 18 ([18F]flortaucipir) in patients with semantic variant primary progressive aphasia (svPPA).
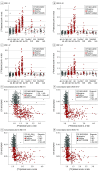
Exposures: [18F]RO948 (all patients) and [18F]flortaucipir (3 patients with svPPA) tau PET; MRI (hippocampal volume, composite temporal lobe cortical thickness, whole-brain cortical thickness) and cerebrospinal fluid measures (p-tau181 and amyloid Aβ42 and Aβ40 ratio[Aβ42/Aβ40], and Aβ42/p-tau181 ratio).
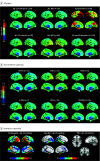
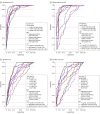
Main outcomes and measures: Standard uptake value ratios (SUVRs) in 4 predefined regions of interest (ROIs) reflecting Braak staging scheme for tau pathology and encompass I-II (entorhinal cortex), III-IV (inferior/middle temporal, fusiform gyrus, parahippocampal cortex, and amygdala), I-IV, and V-VI (widespread neocortical areas), area under the receiver operating characteristic curve (AUC) values, and subtraction images between [18F]RO948 and [18F]flortaucipir.
Results: Diagnostic groups among the 613 participants included cognitively unimpaired (mean [SD] age, 65.8 [12.1] years; 117 men [46%]), mild cognitive impairment (age, 70.8 [8.3] years; 82 men [53%]), AD dementia (age, 73.5 [6.7] years; 57 men [57%]), and non-AD disorders (age, 70.5 [8.6] years; 41 men [40%]). Retention of [18F]RO948 was higher in AD dementia compared with all other diagnostic groups. [18F]RO948 could distinguish patients with AD dementia from individuals without cognitive impairment and those with non-AD disorders, and the highest AUC was obtained using the I-IV ROI (AUC = 0.98; 95% CI, 0.96-0.99 for AD vs no cognitive impairment and AUC = 0.97; 95% CI, 0.95-0.99 for AD vs non-AD disorders), which outperformed MRI (highest AUC = 0.91 for AD vs no cognitive impairment using whole-brain thickness, and AUC = 0.80 for AD vs non-AD disorders using temporal lobe thickness) and cerebrospinal fluid measures (highest AUC = 0.94 for AD vs no cognitive impairment using Aβ42/p-tau181, and AUC = 0.93 for AD vs non-AD disorders using Aβ42/Aβ40). Generally, tau PET positivity using [18F]RO948 was observed only in Aβ-positive cases or in MAPT R406W mutation carriers. Retention of [18F]RO948 was not pronounced in patients with svPPA, and head-to-head comparison revealed lower temporal lobe uptake than with [18F]flortaucipir.
Conclusions and relevance: In this study, elevated [18F]RO948 SUVRs were most often seen among Aβ-positive cases, which suggests that [18F]RO948 has high specificity for AD-type tau and highlights its potential as a diagnostic marker in the differential diagnosis of AD.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

