Current challenges in three-dimensional bioprinting heart tissues for cardiac surgery
- PMID: 32391914
- PMCID: PMC8456486
- DOI: 10.1093/ejcts/ezaa093
Current challenges in three-dimensional bioprinting heart tissues for cardiac surgery
Abstract
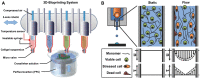
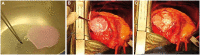
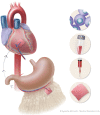
Previous attempts in cardiac bioengineering have failed to provide tissues for cardiac regeneration. Recent advances in 3-dimensional bioprinting technology using prevascularized myocardial microtissues as 'bioink' have provided a promising way forward. This review guides the reader to understand why myocardial tissue engineering is difficult to achieve and how revascularization and contractile function could be restored in 3-dimensional bioprinted heart tissue using patient-derived stem cells.
Keywords: Bioprinting; Cardiac tissues; Regenerative medicine; Revascularization; Stem cells; Transplantation.
© The Author(s) 2020. Published by Oxford University Press on behalf of the European Association for Cardio-Thoracic Surgery.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

