Yersinia pseudotuberculosis YopH targets SKAP2-dependent and independent signaling pathways to block neutrophil antimicrobial mechanisms during infection
- PMID: 32392230
- PMCID: PMC7241846
- DOI: 10.1371/journal.ppat.1008576
Yersinia pseudotuberculosis YopH targets SKAP2-dependent and independent signaling pathways to block neutrophil antimicrobial mechanisms during infection
Abstract
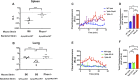
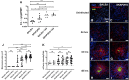
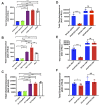
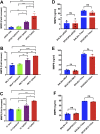
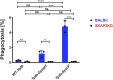
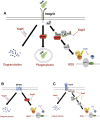
Yersinia suppress neutrophil responses by using a type 3 secretion system (T3SS) to inject 6-7 Yersinia effector proteins (Yops) effectors into their cytoplasm. YopH is a tyrosine phosphatase that causes dephosphorylation of the adaptor protein SKAP2, among other targets in neutrophils. SKAP2 functions in reactive oxygen species (ROS) production, phagocytosis, and integrin-mediated migration by neutrophils. Here we identify essential neutrophil functions targeted by YopH, and investigate how the interaction between YopH and SKAP2 influence Yersinia pseudotuberculosis (Yptb) survival in tissues. The growth defect of a ΔyopH mutant was restored in mice defective in the NADPH oxidase complex, demonstrating that YopH is critical for protecting Yptb from ROS during infection. The growth of a ΔyopH mutant was partially restored in Skap2-deficient (Skap2KO) mice compared to wild-type (WT) mice, while induction of neutropenia further enhanced the growth of the ΔyopH mutant in both WT and Skap2KO mice. YopH inhibited both ROS production and degranulation triggered via integrin receptor, G-protein coupled receptor (GPCR), and Fcγ receptor (FcγR) stimulation. SKAP2 was required for integrin receptor and GPCR-mediated ROS production, but dispensable for degranulation under all conditions tested. YopH blocked SKAP2-independent FcγR-stimulated phosphorylation of the proximal signaling proteins Syk, SLP-76, and PLCγ2, and the more distal signaling protein ERK1/2, while only ERK1/2 phosphorylation was dependent on SKAP2 following integrin receptor activation. These findings reveal that YopH prevents activation of both SKAP2-dependent and -independent neutrophilic defenses, uncouple integrin- and GPCR-dependent ROS production from FcγR responses based on their SKAP2 dependency, and show that SKAP2 is not required for degranulation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

