Coarse-Grained Molecular Model for the Glycosylphosphatidylinositol Anchor with and without Protein
- PMID: 32392421
- PMCID: PMC7303967
- DOI: 10.1021/acs.jctc.0c00056
Coarse-Grained Molecular Model for the Glycosylphosphatidylinositol Anchor with and without Protein
Abstract
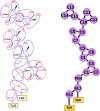
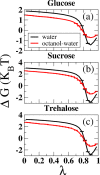
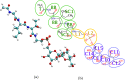
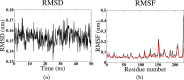
Glycosylphosphatidylinositol (GPI) anchors are a unique class of complex glycolipids that anchor a great variety of proteins to the extracellular leaflet of plasma membranes of eukaryotic cells. These anchors can exist either with or without an attached protein called GPI-anchored protein (GPI-AP) both in vitro and in vivo. Although GPIs are known to participate in a broad range of cellular functions, it is to a large extent unknown how these are related to GPI structure and composition. Their conformational flexibility and microheterogeneity make it difficult to study them experimentally. Simplified atomistic models are amenable to all-atom computer simulations in small lipid bilayer patches but not suitable for studying their partitioning and trafficking in complex and heterogeneous membranes. Here, we present a coarse-grained model of the GPI anchor constructed with a modified version of the MARTINI force field that is suited for modeling carbohydrates, proteins, and lipids in an aqueous environment using MARTINI's polarizable water. The nonbonded interactions for sugars were reparametrized by calculating their partitioning free energies between polar and apolar phases. In addition, sugar-sugar interactions were optimized by adjusting the second virial coefficients of osmotic pressures for solutions of glucose, sucrose, and trehalose to match with experimental data. With respect to the conformational dynamics of GPI-anchored green fluorescent protein, the accessible time scales are now at least an order of magnitude larger than for the all-atom system. This is particularly important for fine-tuning the mutual interactions of lipids, carbohydrates, and amino acids when comparing to experimental results. We discuss the prospective use of the coarse-grained GPI model for studying protein-sorting and trafficking in membrane models.
Conflict of interest statement
The authors declare no competing financial interest.
Figures















Similar articles
-
The importance of side branches of glycosylphosphatidylinositol anchors: a molecular dynamics perspective.Glycobiology. 2022 Oct 31;32(11):933-948. doi: 10.1093/glycob/cwac037. Glycobiology. 2022. PMID: 36197124 Free PMC article.
-
The glycosylphosphatidylinositol anchor: a complex membrane-anchoring structure for proteins.Biochemistry. 2008 Jul 8;47(27):6991-7000. doi: 10.1021/bi8006324. Epub 2008 Jun 17. Biochemistry. 2008. PMID: 18557633 Free PMC article. Review.
-
Synthetic analogues of glycosylphosphatidylinositol-anchored proteins and their behavior in supported lipid bilayers.J Am Chem Soc. 2007 Sep 19;129(37):11543-50. doi: 10.1021/ja073271j. Epub 2007 Aug 23. J Am Chem Soc. 2007. PMID: 17715922
-
Mechanical compressibility of the glycosylphosphatidylinositol (GPI) anchor backbone governed by independent glycosidic linkages.J Am Chem Soc. 2012 Nov 21;134(46):18964-72. doi: 10.1021/ja302803r. Epub 2012 Nov 13. J Am Chem Soc. 2012. PMID: 23061547
-
Membrane insertion and intercellular transfer of glycosylphosphatidylinositol-anchored proteins: potential therapeutic applications.Arch Physiol Biochem. 2020 May;126(2):139-156. doi: 10.1080/13813455.2018.1498904. Epub 2018 Nov 16. Arch Physiol Biochem. 2020. PMID: 30445857 Review.
Cited by
-
Structural basis for membrane attack complex inhibition by CD59.Nat Commun. 2023 Feb 16;14(1):890. doi: 10.1038/s41467-023-36441-z. Nat Commun. 2023. PMID: 36797260 Free PMC article.
-
Supramolecular organization and dynamics of mannosylated phosphatidylinositol lipids in the mycobacterial plasma membrane.Proc Natl Acad Sci U S A. 2023 Jan 31;120(5):e2212755120. doi: 10.1073/pnas.2212755120. Epub 2023 Jan 24. Proc Natl Acad Sci U S A. 2023. PMID: 36693100 Free PMC article.
-
Recent research progress in glycosylphosphatidylinositol-anchored protein biosynthesis, chemical/chemoenzymatic synthesis, and interaction with the cell membrane.Curr Opin Chem Biol. 2024 Feb;78:102421. doi: 10.1016/j.cbpa.2023.102421. Epub 2024 Jan 4. Curr Opin Chem Biol. 2024. PMID: 38181647 Free PMC article. Review.
References
-
- Tachado S. D.; Gerold P.; Schwarz R.; Novakovic S.; McConville M.; Schofield L. Signal transduction in macrophages by glycosylphosphatidylinositols of Plasmodium, Trypanosoma, and Leishmania: activation of protein tyrosine kinases and protein kinase C by inositolglycan and diacylglycerol moieties. Proc. Natl. Acad. Sci. U. S. A. 1997, 94, 4022–4027. 10.1073/pnas.94.8.4022. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

