The DNA Damage Response and HIV-Associated Pulmonary Arterial Hypertension
- PMID: 32392789
- PMCID: PMC7246454
- DOI: 10.3390/ijms21093305
The DNA Damage Response and HIV-Associated Pulmonary Arterial Hypertension
Abstract
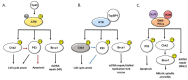
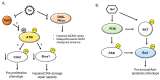
The HIV-infected population is at a dramatically increased risk of developing pulmonary arterial hypertension (PAH), a devastating and fatal cardiopulmonary disease that is rare amongst the general population. It is increasingly apparent that PAH is a disease with complex and heterogeneous cellular and molecular pathologies, and options for therapeutic intervention are limited, resulting in poor clinical outcomes for affected patients. A number of soluble HIV factors have been implicated in driving the cellular pathologies associated with PAH through perturbations of various signaling and regulatory networks of uninfected bystander cells in the pulmonary vasculature. While these mechanisms are likely numerous and multifaceted, the overlapping features of PAH cellular pathologies and the effects of viral factors on related cell types provide clues as to the potential mechanisms driving HIV-PAH etiology and progression. In this review, we discuss the link between the DNA damage response (DDR) signaling network, chronic HIV infection, and potential contributions to the development of pulmonary arterial hypertension in chronically HIV-infected individuals.
Keywords: DNA damage; HIV; Nef; Tat; endothelial; pulmonary arterial hypertension.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Aldred M.A., Comhair S.A., Varella-Garcia M., Asosingh K., Xu W., Noon G.P., Thistlethwaite P.A., Tuder R.M., Erzurum S.C., Geraci M.W., et al. Somatic chromosome abnormalities in the lungs of patients with pulmonary arterial hypertension. Am. J. Respir. Crit. Care. 2010;182:1153–1160. doi: 10.1164/rccm.201003-0491OC. - DOI - PMC - PubMed
-
- Almodovar S., Knight R., Allshouse A.A., Roemer S., Lozupone C., McDonald D., Widmann J., Voelkel N.F., Shelton R.J., Suarez E.B., et al. Human Immunodeficiency Virus nef signature sequences are associated with pulmonary hypertension. AIDS Res. Hum. Retrovir. 2012;28:607–618. doi: 10.1089/aid.2011.0021. - DOI - PMC - PubMed
-
- Barnett C.F., Hsue P.Y. HIV-Associated Pulmonary Hypertension: A Global Perspective. Adv. Pulm. Hypertens. 2017;15:138–143. doi: 10.21693/1933-088X-15.3.138. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

