The Potential Functional Roles of NME1 Histidine Kinase Activity in Neuroblastoma Pathogenesis
- PMID: 32392889
- PMCID: PMC7247550
- DOI: 10.3390/ijms21093319
The Potential Functional Roles of NME1 Histidine Kinase Activity in Neuroblastoma Pathogenesis
Abstract
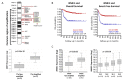
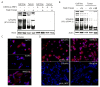
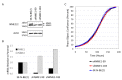
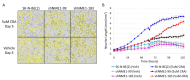
Neuroblastoma is the most common extracranial solid tumor in childhood. Gain of chromosome 17q material is found in >60% of neuroblastoma tumors and is associated with poor patient prognosis. The NME1 gene is located in the 17q21.3 region, and high NME1 expression is correlated with poor neuroblastoma patient outcomes. However, the functional roles and signaling activity of NME1 in neuroblastoma cells and tumors are unknown. NME1 and NME2 have been shown to possess histidine (His) kinase activity. Using anti-1- and 3-pHis specific monoclonal antibodies and polyclonal anti-pH118 NME1/2 antibodies, we demonstrated the presence of pH118-NME1/2 and multiple additional pHis-containing proteins in all tested neuroblastoma cell lines and in xenograft neuroblastoma tumors, supporting the presence of histidine kinase activity in neuroblastoma cells and demonstrating the potential significance of histidine kinase signaling in neuroblastoma pathogenesis. We have also demonstrated associations between NME1 expression and neuroblastoma cell migration and differentiation. Our demonstration of NME1 histidine phosphorylation in neuroblastoma and of the potential role of NME1 in neuroblastoma cell migration and differentiation suggest a functional role for NME1 in neuroblastoma pathogenesis and open the possibility of identifying new therapeutic targets and developing novel approaches to neuroblastoma therapy.
Keywords: NME/NM23/NDPK; histidine; kinase; neuroblastoma; phosphorylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Bown N., Lastowska M., Cotterill S., O’Neill S., Ellershaw C., Roberts P., Lewis I., Pearson A.D., U.K. Cancer Cytogenetics Group and the U.K. Children’s Cancer Study Group 17q gain in neuroblastoma predicts adverse clinical outcome. Med. Pediatr. Oncol. 2001;36:14–19. doi: 10.1002/1096-911X(20010101)36:1<14::AID-MPO1005>3.0.CO;2-G. - DOI - PubMed
-
- Meddeb M., Danglot G., Chudoba I., Vénuat A.M., Bénard J., Avet-Loiseau H., Vasseur B., Le Paslier D., Terrier-Lacombe M.J., Hartmann O., et al. Additional copies of a 25 Mb chromosomal region originating from 17q23.1-17qter are present in 90% of high-grade neuroblastomas. Genes Chromosomes Cancer. 1996;17:156–165. doi: 10.1002/(SICI)1098-2264(199611)17:3<156::AID-GCC3>3.0.CO;2-3. - DOI - PubMed
-
- Bown N., Cotterill S., Lastowska M., O’Neill S., Pearson A.D., Plantaz D., Meddeb M., Danglot G., Brinkschmidt C., Christiansen H., et al. Gain of chromosome arm 17q and adverse outcome in patients with neuroblastoma. N. Engl. J. Med. 1999;340:1954–1961. doi: 10.1056/NEJM199906243402504. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

