Ceramide and Sphingosine 1-Phosphate in Liver Diseases
- PMID: 32392908
- PMCID: PMC7264474
- DOI: 10.14348/molcells.2020.0054
Ceramide and Sphingosine 1-Phosphate in Liver Diseases
Abstract
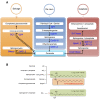
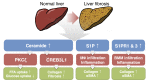
The liver is an important organ in the regulation of glucose and lipid metabolism. It is responsible for systemic energy homeostasis. When energy need exceeds the storage capacity in the liver, fatty acids are shunted into nonoxidative sphingolipid biosynthesis, which increases the level of cellular ceramides. Accumulation of ceramides alters substrate utilization from glucose to lipids, activates triglyceride storage, and results in the development of both insulin resistance and hepatosteatosis, increasing the likelihood of major metabolic diseases. Another sphingolipid metabolite, sphingosine 1-phosphate (S1P) is a bioactive signaling molecule that acts via S1P-specific G protein coupled receptors. It regulates many cellular and physiological events. Since an increase in plasma S1P is associated with obesity, it seems reasonable that recent studies have provided evidence that S1P is linked to lipid pathophysiology, including hepatosteatosis and fibrosis. Herein, we review recent findings on ceramides and S1P in obesity-mediated liver diseases and the therapeutic potential of these sphingolipid metabolites.
Keywords: ceramide; fibrosis; insulin resistance; obesity; sphingosine 1-phosphate; steatosis.
Conflict of interest statement
The authors have no potential conflicts of interest to disclose.
Figures
References
-
- Barr E.L., Cameron A.J., Balkau B., Zimmet P.Z., Welborn T.A., Tonkin A.M., Shaw J.E. HOMA insulin sensitivity index and the risk of all-cause mortality and cardiovascular disease events in the general population: the Australian Diabetes, Obesity and Lifestyle Study (AusDiab) study. Diabetologia. 2010;53:79–88. doi: 10.1007/s00125-009-1588-0. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

