Reactive cutaneous capillary endothelial proliferation in advanced hepatocellular carcinoma patients treated with camrelizumab: data derived from a multicenter phase 2 trial
- PMID: 32393323
- PMCID: PMC7216554
- DOI: 10.1186/s13045-020-00886-2
Reactive cutaneous capillary endothelial proliferation in advanced hepatocellular carcinoma patients treated with camrelizumab: data derived from a multicenter phase 2 trial
Abstract
Background: Association of immune-related adverse events with tumor response has been reported. Reactive cutaneous capillary endothelial proliferation (RCCEP) is the most common adverse event related to camrelizumab, an immune checkpoint inhibitor, but lack of comprehensive analyses. In this study, we conducted comprehensive analyses on RCCEP in advanced hepatocellular carcinoma (HCC) patients treated with camrelizumab monotherapy.
Methods: Data were derived from a Chinese nationwide, multicenter phase 2 trial of camrelizumab in pre-treated advanced HCC. The occurrence, clinicopathological characteristics, and prognostic value of RCCEP were analyzed.
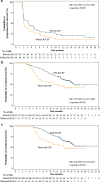
Results: With a median follow-up of 12.5 months, 145 of the 217 camrelizumab-treated patients (66.8%) experienced RCCEP (all grade 1 or 2). RCCEP occurred on the skin surface, mainly on the skin surface of head, face, and trunk. RCCEP could be divided into 5 types including "red-nevus-like," "pearl-like," "mulberry-like," "patch-like," and "tumor-like," according to the morphological features. RCCEP biopsy and pathology showed capillary endothelial hyperplasia and capillary hyperplasia in dermis. Significant association between RCCEP occurrence with higher objective response rate was observed (19.3% vs. 5.6%; one-sided p = 0.0044). Compared with those without RCCEP, patients with RCCEP had prolonged progression-free survival (median PFS; 3.2 months vs. 1.9 months; one-sided p < 0.0001) and overall survival (median OS; 17.0 months vs. 5.8 months; one-sided p < 0.0001). In multivariable analyses, the development of RCCEP was significantly associated with prolonged PFS and OS after adjusting for baseline covariates. In addition, the landmark analyses of PFS and OS were consistent with the unadjusted analysis.
Conclusions: RCCEP occurred on the skin surface and was an immune response of skin capillary endothelial cells. RCCEP occurrence positively associated with outcomes of camrelizumab in advanced HCC.
Keywords: Anti–PD-1 antibody; Camrelizumab; Hepatocellular carcinoma; Immune-related adverse events; Reactive cutaneous capillary endothelial proliferation.
Conflict of interest statement
Linna Wang, Ping Yan, and Jianjun Zou are employees of Jiangsu Hengrui Medicine Co., Ltd. Other co-authors declare no competing interests.
Figures




Similar articles
-
Predicting Outcome in Combination Treatment of TACE and Camrelizumab for Advanced Hepatocellular carcinoma: Tumor Hypervascularity and Reactive Cutaneous Capillary Endothelial Proliferation.Drug Des Devel Ther. 2022 Sep 30;16:3421-3429. doi: 10.2147/DDDT.S372276. eCollection 2022. Drug Des Devel Ther. 2022. PMID: 36203820 Free PMC article.
-
Association between reactive cutaneous capillary endothelial proliferation and the efficacy of camrelizumab in esophageal cancer: a retrospective cohort study.J Thorac Dis. 2025 Apr 30;17(4):2453-2472. doi: 10.21037/jtd-2025-366. Epub 2025 Apr 28. J Thorac Dis. 2025. PMID: 40400922 Free PMC article.
-
Reactive cutaneous capillary endothelial proliferation predicted the efficacy of camrelizumab in patients with recurrent/metastatic head and neck squamous cell carcinoma.Med Oral Patol Oral Cir Bucal. 2023 Nov 1;28(6):e525-e529. doi: 10.4317/medoral.25919. Med Oral Patol Oral Cir Bucal. 2023. PMID: 37330963 Free PMC article.
-
The clinical application of camrelizumab on advanced hepatocellular carcinoma.Expert Rev Gastroenterol Hepatol. 2020 Nov;14(11):1017-1024. doi: 10.1080/17474124.2020.1807939. Epub 2020 Aug 18. Expert Rev Gastroenterol Hepatol. 2020. PMID: 32762583 Review.
-
Experience With Anti-PD-1 Antibody, Camrelizumab, Monotherapy for Biliary Tract Cancer Patients and Literature Review.Technol Cancer Res Treat. 2020 Jan-Dec;19:1533033820979703. doi: 10.1177/1533033820979703. Technol Cancer Res Treat. 2020. PMID: 33308041 Free PMC article. Review.
Cited by
-
Lenvatinib Plus Camrelizumab versus Lenvatinib Monotherapy as Post-Progression Treatment for Advanced Hepatocellular Carcinoma: A Short-Term Prognostic Study.Cancer Manag Res. 2021 May 27;13:4233-4240. doi: 10.2147/CMAR.S304820. eCollection 2021. Cancer Manag Res. 2021. PMID: 34079375 Free PMC article.
-
Case report: A case of acute mastitis associated with reactive cutaneous capillary endothelial proliferation after camrelizumab treatment: A new immune-related adverse event.Front Immunol. 2022 Aug 25;13:939873. doi: 10.3389/fimmu.2022.939873. eCollection 2022. Front Immunol. 2022. PMID: 36090986 Free PMC article.
-
Toxicity profile of combined immune checkpoint inhibitors and thoracic radiotherapy in esophageal cancer: A meta-analysis and systematic review.Front Immunol. 2022 Nov 10;13:1039020. doi: 10.3389/fimmu.2022.1039020. eCollection 2022. Front Immunol. 2022. PMID: 36439117 Free PMC article.
-
Camrelizumab for the treatment of advanced cervical adenocarcinoma: a case report and literature review.Ann Transl Med. 2022 Feb;10(4):239. doi: 10.21037/atm-22-67. Ann Transl Med. 2022. PMID: 35280424 Free PMC article.
-
Research Progress of Biomarkers for Immune Checkpoint Inhibitors on Digestive System Cancers.Front Immunol. 2022 Apr 13;13:810539. doi: 10.3389/fimmu.2022.810539. eCollection 2022. Front Immunol. 2022. PMID: 35493526 Free PMC article. Review.
References
-
- Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–1920. doi: 10.1016/S0140-6736(16)00561-4. - DOI - PMC - PubMed
-
- Kaufman HL, Russell J, Hamid O, Bhatia S, Terheyden P, D'Angelo SP, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17:1374–1385. doi: 10.1016/S1470-2045(16)30364-3. - DOI - PMC - PubMed
-
- Planchard D, Yokoi T, McCleod MJ, Fischer JR, Kim YC, Ballas M, et al. A Phase III Study of Durvalumab (MEDI4736) With or without tremelimumab for previously treated patients with advanced NSCLC: rationale and protocol design of the ARCTIC study. Clin Lung Cancer. 2016;17:232–236. doi: 10.1016/j.cllc.2016.03.003. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

