Sex differences in traumatic stress reactivity in rats with and without a history of alcohol drinking
- PMID: 32393336
- PMCID: PMC7216391
- DOI: 10.1186/s13293-020-00303-w
Sex differences in traumatic stress reactivity in rats with and without a history of alcohol drinking
Abstract
Background: Alcohol misuse and post-traumatic stress disorder (PTSD) are highly comorbid, and treatment outcomes are worse in individuals with both conditions. Although more men report experiencing traumatic events than women, the lifetime prevalence of PTSD is twice as high in females. Despite these data trends in humans, preclinical studies of traumatic stress reactivity have been performed almost exclusively in male animals.
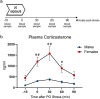
Methods: This study was designed to examine sex differences in traumatic stress reactivity in alcohol-naive rats (experiment 1) and rats given intermittent access to 20% ethanol in a 2-bottle choice paradigm for 5 weeks (experiment 2). Animals were exposed to predator odor (bobcat urine) and tested for contextual avoidance 24 h later; unstressed controls were never exposed to predator odor. We evaluated changes in physiological arousal using the acoustic startle response (ASR) test at day 2 post-stress and anxiety-like behavior measured in the elevated plus-maze (EPM) at day 17 post-stress. In experiment 3, time course of corticosterone response was examined in male and female rats following exposure to predator odor stress.
Results: Alcohol-naive males and females exposed to predator odor displayed blunted weight gain 24 h post-stress, but only a subset of stressed animals exhibited avoidance behavior. In alcohol-drinking animals, the proportion of avoiders was higher in males than females, and predator odor exposure increased ASR in males only. Stressed females exhibited blunted ASR relative to unstressed females and stressed males, regardless of alcohol drinking history. Alcohol-experienced females presented lower anxiety-like behavior and higher general activity in the EPM in comparison with alcohol-experienced males. Plasma corticosterone levels were higher in females immediately after predator odor exposure until 60 min post-stress relative to males.
Conclusions: We report robust sex differences in behavioral and endocrine responses to bobcat urine exposure in adult Wistar rats. Also, males with a history of chronic moderate alcohol drinking exhibited increased traumatic stress reactivity relative to alcohol-drinking females. Our findings emphasize the importance of considering sex as a biological variable in the investigation of traumatic stress effects on physiology and behavior.
Keywords: Alcohol; Arousal; Bobcat urine; Corticosterone; Predator odor; Sex differences; Startle; Stress; Trauma.
Conflict of interest statement
NWG owns shares in Glauser Life Sciences, a company with interest in developing therapeutics for mental health disorders. There is no direct link between those interests and the work contained herein.
Figures


References
-
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5. Arlington: American Psychiatric Publishing; 2013.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

