The impact of glutamate infusion on postoperative NT-proBNP in patients undergoing coronary artery bypass surgery: a randomized study
- PMID: 32393387
- PMCID: PMC7216679
- DOI: 10.1186/s12967-020-02351-7
The impact of glutamate infusion on postoperative NT-proBNP in patients undergoing coronary artery bypass surgery: a randomized study
Abstract
Background: Glutamate, a key intermediate in myocardial metabolism, may enhance myocardial recovery after ischemia and possibly reduce the incidence and severity of postoperative heart failure in coronary artery bypass surgery (CABG). N-terminal pro-B-type natriuretic peptide (NT-proBNP) can be used to assess postoperative heart failure (PHF) after CABG. Our hypothesis was that glutamate enhances myocardial recovery in post-ischemic heart failure and, therefore, will be accompanied by a mitigated postoperative increase of NT-proBNP.
Methods: Substudy of the GLUTAmate for Metabolic Intervention in Coronary Surgery (GLUTAMICS) trial (ClinicalTrials.gov Identifier: NCT00489827) a prospective triple-center double-blind randomized clinical trial on 399 patients undergoing CABG with or without concomitant procedure for acute coronary syndrome at three Swedish Cardiac Surgery centres (Linköping, Örebro, and Karlskrona) from May 30, 2007 to November 12, 2009. Patients were randomly assigned to intravenous infusion of 0.125 M L-glutamic acid or saline (1.65 mL/kg of body weight per hour) intraoperatively and postoperatively. Plasma NT-proBNP was measured preoperatively, the first (POD1) and third postoperative morning (POD3). A Clinical Endpoints Committee, blinded to both intervention and NT-proBNP used prespecified criteria to diagnose PHF. The primary endpoints were the absolute levels of postoperative NT-proBNP and the difference between preoperative and postoperative levels of NT-proBNP.
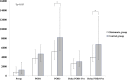
Results: Overall no significant difference was detected in postoperative NT-proBNP levels between groups. However, in high-risk patients (upper quartile of EuroSCORE II ≥ 4.15; glutamate group n = 56; control group n = 45) glutamate was associated with significantly lower postoperative increase of NT-proBNP (POD3-Pre: 3900 [2995-6260] vs. 6745 [3455-12,687] ng•L-1, p = 0.012) and lower NT-proBNP POD3 (POD3: 4845 [3426-7423] vs. 8430 [5370-14,100] ng•L-1, p = 0.001). After adjusting for significant differences in preoperative demographics, NT-proBNP POD3 in the glutamate group was 0.62 times of that in the control group (p = 0.002). Patients in the glutamate group also had shorter ICU stay (21 [19-26] vs. 25 [22-46] h, p = 0.025) and less signs of myocardial injury (Troponin T POD3 (300 [170-500] vs. 560 [210-910] ng•L-1, p = 0.025).
Conclusions: Post hoc analysis of postoperative NT-proBNP suggests that intravenous infusion of glutamate may prevent or mitigate myocardial dysfunction in high-risk patients undergoing CABG. Further studies are necessary to confirm these findings. Trial registration Swedish Medical Products Agency 151:2003/70403 (prospectively registered with amendment about this substudy filed March 17, 2007). ClinicalTrials.gov Identifier: NCT00489827 (retrospectively registered) https://clinicaltrials.gov/ct2/show/NCT00489827?term=glutamics&draw=1&rank=1.
Keywords: Coronary artery bypass surgery; Glutamic acid; Heart failure; Natriuretic peptide; Postoperative care.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Effect of glutamate infusion on NT-proBNP after coronary artery bypass grafting in high-risk patients (GLUTAMICS II): A randomized controlled trial.PLoS Med. 2022 May 9;19(5):e1003997. doi: 10.1371/journal.pmed.1003997. eCollection 2022 May. PLoS Med. 2022. PMID: 35533197 Free PMC article. Clinical Trial.
-
Utility of NT-proBNP as an objective marker of postoperative heart failure after coronary artery bypass surgery: a prospective observational study.Perioper Med (Lond). 2021 Jul 13;10(1):21. doi: 10.1186/s13741-021-00194-4. Perioper Med (Lond). 2021. PMID: 34253255 Free PMC article.
-
Glutamate Infusion Reduces Myocardial Dysfunction after Coronary Artery Bypass Grafting According to NT-proBNP: Summary of 2 Randomized Controlled Trials (GLUTAmate for Metabolic Intervention in Coronary Surgery [GLUTAMICS I-II]).Am J Clin Nutr. 2023 Nov;118(5):930-937. doi: 10.1016/j.ajcnut.2023.08.012. Epub 2023 Aug 30. Am J Clin Nutr. 2023. PMID: 37657522
-
EuroSCORE II and N-terminal pro-B-type natriuretic peptide for risk evaluation: an observational longitudinal study in patients undergoing coronary artery bypass graft surgery.Br J Anaesth. 2014 Jul;113(1):75-82. doi: 10.1093/bja/aeu088. Epub 2014 Apr 11. Br J Anaesth. 2014. PMID: 24727704
-
Preoperative B-Type Natriuretic Peptides to Predict Postoperative Atrial Fibrillation in Cardiac Surgery: A Systematic Review and Meta-Analysis.Heart Lung Circ. 2024 Jan;33(1):23-32. doi: 10.1016/j.hlc.2023.10.015. Epub 2023 Dec 23. Heart Lung Circ. 2024. PMID: 38143193
Cited by
-
The Role of Cardiac N-Methyl-D-Aspartate Receptors in Heart Conditioning-Effects on Heart Function and Oxidative Stress.Biomolecules. 2020 Jul 16;10(7):1065. doi: 10.3390/biom10071065. Biomolecules. 2020. PMID: 32708792 Free PMC article.
-
Plasma Amino Acid Concentrations at Birth and Patent Ductus Arteriosus in Very and Extremely Preterm Infants.Front Pediatr. 2021 Feb 11;9:647018. doi: 10.3389/fped.2021.647018. eCollection 2021. Front Pediatr. 2021. PMID: 33643980 Free PMC article.
-
Effect of glutamate infusion on NT-proBNP after coronary artery bypass grafting in high-risk patients (GLUTAMICS II): A randomized controlled trial.PLoS Med. 2022 May 9;19(5):e1003997. doi: 10.1371/journal.pmed.1003997. eCollection 2022 May. PLoS Med. 2022. PMID: 35533197 Free PMC article. Clinical Trial.
References
-
- O’Connor GT, Birkmeyer JD, Dacey LJ, Quinton HB, Marrin CA, Birkmeyer NJ, Morton JR, Leavitt BJ, Maloney CT, Hernandez F, Clough RA, Nugent WC, Olmstead EM, Charlesworth DC, Plume SK. Results of a regional study of modes of death associated with coronary artery bypass grafting. Northern New England Cardiovascular Disease Study Group. Ann Thorac Surg. 1998;66:1323–1328. doi: 10.1016/S0003-4975(98)00762-0. - DOI - PubMed
-
- Surgenor SD, O’Connor GT, Lahey SJ, Quinn R, Charlesworth DC, Dacey LJ, Clough RA, Leavitt BJ, Defoe GR, Fillinger M, Nugent WC, Northern New England Cardiovascular Disease Study G Predicting the risk of death from heart failure after coronary artery bypass graft surgery. Anesth Analg. 2001;92:596–601. doi: 10.1213/00000539-200103000-00008. - DOI - PubMed
-
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force M, Document R 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. - DOI - PubMed
-
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. J Card Fail. 2017;23:628–651. doi: 10.1016/j.cardfail.2017.04.014. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous