Downregulation of p16 Decreases Biliary Damage and Liver Fibrosis in the Mdr2/ Mouse Model of Primary Sclerosing Cholangitis
- PMID: 32393417
- PMCID: PMC7650011
- DOI: 10.3727/105221620X15889714507961
Downregulation of p16 Decreases Biliary Damage and Liver Fibrosis in the Mdr2/ Mouse Model of Primary Sclerosing Cholangitis
Abstract
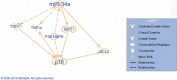
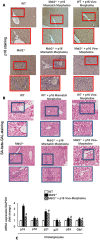
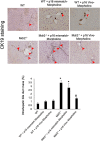
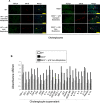
Biliary senescence and hepatic fibrosis are hallmarks of cholangiopathies including primary sclerosing cholangitis (PSC). Senescent cholangiocytes display senescence-associated secretory phenotypes [SASPs, e.g., transforming growth factor-1 (TGF-1)] that further increase biliary senescence (by an autocrine loop) and trigger liver fibrosis by paracrine mechanisms. The aim of this study was to determine the effect of p16 inhibition and role of the TGF-1/microRNA (miR)-34a/sirtuin 1 (SIRT1) axis in biliary damage and liver fibrosis in the Mdr2/ mouse model of PSC. We treated (i) in vivo male wild-type (WT) and Mdr2/ mice with p16 Vivo-Morpholino or controls before measuring biliary mass [intrahepatic bile duct mass (IBDM)] and senescence, biliary SASP levels, and liver fibrosis, and (ii) in vitro intrahepatic murine cholangiocyte lines (IMCLs) with small interfering RNA against p16 before measuring the mRNA expression of proliferation, senescence, and fibrosis markers. p16 and miR-34a increased but SIRT1 decreased in Mdr2/ mice and PSC human liver samples compared to controls. p16 immunoreactivity and biliary senescence and SASP levels increased in Mdr2/ mice but decreased in Mdr2/ mice treated with p16 Vivo-Morpholino. The increase in IBDM and hepatic fibrosis (observed in Mdr2/ mice) returned to normal values in Mdr2/ mice treated with p16 Vivo-Morpholino. TGF-1 immunoreactivity and biliary SASPs levels were higher in Mdr2/ compared to those of WT mice but returned to normal values in Mdr2/ mice treated with p16 Vivo-Morpholino. The expression of fibrosis/senescence markers decreased in cholangiocytes from Mdr2/ mice treated with p16 Vivo-Morpholino (compared to Mdr2/ mice) and in IMCLs (after p16 silencing) compared to controls. Modulation of the TGF-1/miR-34a/SIRT1 axis may be important in the management of PSC phenotypes.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
- R21 AA025157/AA/NIAAA NIH HHS/United States
- R01 DK062975/DK/NIDDK NIH HHS/United States
- R01 DK107310/DK/NIDDK NIH HHS/United States
- I01 BX000574/BX/BLRD VA/United States
- I01 BX003031/BX/BLRD VA/United States
- IK6 BX004601/BX/BLRD VA/United States
- R01 DK108959/DK/NIDDK NIH HHS/United States
- K01 AA026385/AA/NIAAA NIH HHS/United States
- R01 DK119421/DK/NIDDK NIH HHS/United States
- R21 AA025997/AA/NIAAA NIH HHS/United States
- R01 DK110035/DK/NIDDK NIH HHS/United States
- R01 DK054811/DK/NIDDK NIH HHS/United States
- I01 BX001724/BX/BLRD VA/United States
- R01 DK076898/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
