Comparison of a new versus standard removable offloading device in patients with neuropathic diabetic foot ulcers: a French national, multicentre, open-label randomized, controlled trial
- PMID: 32393479
- PMCID: PMC7223015
- DOI: 10.1136/bmjdrc-2019-000954
Comparison of a new versus standard removable offloading device in patients with neuropathic diabetic foot ulcers: a French national, multicentre, open-label randomized, controlled trial
Abstract
Introduction: The offloading is crucial to heal neuropathic diabetic foot ulcer (DFU). Removable offloading are the most used devices. Orthèse diabète is a new customized removable knee-high offloading device immobilizing foot and ankle joints, with some specific and innovative features that may improve offloading. We aimed to evaluate the efficiency of this device in DFU healing.
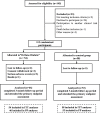
Research, design and methods: The evaluation of Offloading using a new removable ORTHOsis in DIABetic foot study is a French multicenter (13 centers) randomized controlled trial with blinded end points evaluation. Adults with neuropathic DFU were randomly assigned to either Orthèse Diabète (experimental device), or any type of conventional (usually used in France) removable offloading devices (control group). The primary outcome was the 3-month proportion of patients with fully healed DFU.
Results: Among 112 randomized patients (men 78%, age 62±10 years), the primary outcome occurred in 19 (33%) participants using conventional device vs 19 (35%) Orthèse Diabète users (p=0.79). Study groups were also comparable in terms of prespecified secondary end points including occurrence of new DFU (25% vs 27% in conventional and experimental groups), ipsilateral lower-limb amputation (4% vs 10%) or infectious complications (14% vs 13%) (p>0.05 for all). Adverse events were comparable between groups, including 4 deaths unrelated to study allocation (1 sudden death, 2 ventricular arrhythmias and 1 pancreatic cancer). Adverse events believed to be related to the device were higher in the Orthèse Diabète group than in the control group (15% vs 4%). Orthèse Diabète was less frequently worn than conventional devices (46% vs 66%, p=0.04).
Conclusions: Orthèse Diabète, a new removable offloading orthosis immobilizing foot and ankle joints did not show superiority compared with conventional removable devices in neuropathic DFU healing and cannot be recommended to heal DFU.
Trial registration number: NCT01956162.
Keywords: foot ulcer; off-loading devices; randomized clinical trials; wound healing.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: LP reports receiving personal fees from Novo Nordisk, Lilly, Sanofi and Servier and grants from Sanofi, outside the submitted work; RR is an advisory panel member for AstraZeneca, Sanofi, MSD, Eli Lilly, Novo Nordisk, Vaiomer and Physiogenex; is a speaker for Bayer and Servier and has received research funding and provided research support to Danone Research, Diabnext, Boehringer-Ingelheim, Amgen, Sanofi and Novo Nordisk. MM reports receiving personal fees from Novo Nordisk, Sanofi, Eli Lilly, Servier, Merck, Sharp and Dohme, Abbott, Novartis and AstraZeneca and grant support from Novo Nordisk, Sanofi, Eli Lilly, Merck Sharp and Dohme and Novartis, outside the submitted work; KM reports receiving personal fees from Novo Nordisk, Sanofi, Eli Lilly, Proteor and AstraZeneca, and travel support from Novo Nordisk, Sanofi, Boehringer Ingelheim and Takeda outside the submitted work.
Figures
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical