Cellular Stoichiometry of Chemotaxis Proteins in Sinorhizobium meliloti
- PMID: 32393521
- PMCID: PMC7317046
- DOI: 10.1128/JB.00141-20
Cellular Stoichiometry of Chemotaxis Proteins in Sinorhizobium meliloti
Abstract
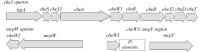
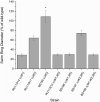
Chemotaxis systems enable microbes to sense their immediate environment, moving toward beneficial stimuli and away from those that are harmful. In an effort to better understand the chemotaxis system of Sinorhizobium meliloti, a symbiont of the legume alfalfa, the cellular stoichiometries of all ten chemotaxis proteins in S. meliloti were determined. A combination of quantitative immunoblot and mass spectrometry revealed that the protein stoichiometries in S. meliloti varied greatly from those in Escherichia coli and Bacillus subtilis To compare protein ratios to other systems, values were normalized to the central kinase CheA. All S. meliloti chemotaxis proteins exhibited increased ratios to various degrees. The 10-fold higher molar ratio of adaptor proteins CheW1 and CheW2 to CheA might result in the formation of rings in the chemotaxis array that consist of only CheW instead of CheA and CheW in a 1:1 ratio. We hypothesize that the higher ratio of CheA to the main response regulator CheY2 is a consequence of the speed-variable motor in S. meliloti, instead of a switch-type motor. Similarly, proteins involved in signal termination are far more abundant in S. meliloti, which utilizes a phosphate sink mechanism based on CheA retrophosphorylation to inactivate the motor response regulator versus CheZ-catalyzed dephosphorylation as in E. coli and B. subtilis Finally, the abundance of CheB and CheR, which regulate chemoreceptor methylation, was increased compared to CheA, indicative of variations in the adaptation system of S. meliloti Collectively, these results mark significant differences in the composition of bacterial chemotaxis systems.IMPORTANCE The symbiotic soil bacterium Sinorhizobium meliloti contributes greatly to host-plant growth by fixing atmospheric nitrogen. The provision of nitrogen as ammonium by S. meliloti leads to increased biomass production of its legume host alfalfa and diminishes the use of environmentally harmful chemical fertilizers. To better understand the role of chemotaxis in host-microbe interaction, a comprehensive catalogue of the bacterial chemotaxis system is vital, including its composition, function, and regulation. The stoichiometry of chemotaxis proteins in S. meliloti has very few similarities to the systems in Escherichia coli and Bacillus subtilis In addition, total amounts of proteins are significantly lower. S. meliloti exhibits a chemotaxis system distinct from known models by incorporating new proteins as exemplified by the phosphate sink mechanism.
Keywords: chemoreceptor methylation; flagellar motility; plant symbiont; rhizosphere; two-component system.
Copyright © 2020 American Society for Microbiology.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

