Looking Down on NF-κB
- PMID: 32393609
- PMCID: PMC7364044
- DOI: 10.1128/MCB.00104-20
Looking Down on NF-κB
Abstract
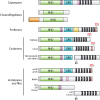
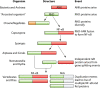
The diversified NF-κB transcription factor family has been extensively characterized in organisms ranging from flies to humans. However, homologs of NF-κB and many upstream signaling components have recently been characterized in basal phyla, including Cnidaria (sea anemones, corals, hydras, and jellyfish), Porifera (sponges), and single-celled protists, including Capsaspora owczarzaki and some choanoflagellates. Herein, we review what is known about basal NF-κBs and how that knowledge informs on the evolution and conservation of key sequences and domains in NF-κB, as well as the regulation of NF-κB activity. The structures and DNA-binding activities of basal NF-κB proteins resemble those of mammalian NF-κB p100 proteins, and their posttranslational activation appears to have aspects of both canonical and noncanonical pathways in mammals. Several studies suggest that the single NF-κB proteins found in some basal organisms have dual roles in development and immunity. Further research on NF-κB in invertebrates will reveal information about the evolutionary roots of this major signaling pathway, will shed light on the origins of regulated innate immunity, and may have relevance to our understanding of the responses of ecologically important organisms to changing environmental conditions and emerging pathogen-based diseases.
Keywords: NF-kappaB; development; evolution; innate immunity; signal transduction.
Copyright © 2020 American Society for Microbiology.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
