Prefrontal-hippocampal functional connectivity encodes recognition memory and is impaired in intellectual disability
- PMID: 32393630
- PMCID: PMC7261130
- DOI: 10.1073/pnas.1921314117
Prefrontal-hippocampal functional connectivity encodes recognition memory and is impaired in intellectual disability
Abstract
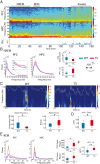
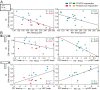
Down syndrome (DS) is the most common form of intellectual disability. The cognitive alterations in DS are thought to depend on brain regions critical for learning and memory such as the prefrontal cortex (PFC) and the hippocampus (HPC). Neuroimaging studies suggest that increased brain connectivity correlates with lower intelligence quotients (IQ) in individuals with DS; however, its contribution to cognitive impairment is unresolved. We recorded neural activity in the PFC and HPC of the trisomic Ts65Dn mouse model of DS during quiet wakefulness, natural sleep, and the performance of a memory test. During rest, trisomic mice showed increased theta oscillations and cross-frequency coupling in the PFC and HPC while prefrontal-hippocampal synchronization was strengthened, suggesting hypersynchronous local and cross-regional processing. During sleep, slow waves were reduced, and gamma oscillations amplified in Ts65Dn mice, likely reflecting prolonged light sleep. Moreover, hippocampal sharp-wave ripples were disrupted, which may have further contributed to deficient memory consolidation. Memory performance in euploid mice correlated strongly with functional connectivity measures that indicated a hippocampal control over memory acquisition and retrieval at theta and gamma frequencies, respectively. By contrast, trisomic mice exhibited poor memory abilities and disordered prefrontal-hippocampal functional connectivity. Memory performance and key neurophysiological alterations were rescued after 1 month of chronic administration of a green tea extract containing epigallocatequin-3-gallate (EGCG), which improves executive function in young adults with DS and Ts65Dn mice. Our findings suggest that abnormal prefrontal-hippocampal circuit dynamics are candidate neural mechanisms for memory impairment in DS.
Keywords: Down syndrome; functional connectivity; intellectual disability; learning and memory; neural networks.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Dierssen M., Down syndrome: The brain in trisomic mode. Nat. Rev. Neurosci. 13, 844–858 (2012). - PubMed
-
- Dierssen M., et al. , Alterations of neocortical pyramidal cell phenotype in the Ts65Dn mouse model of Down syndrome: Effects of environmental enrichment. Cereb. Cortex 13, 758–764 (2003). - PubMed
-
- Davisson M. T., et al. , Segmental trisomy as a mouse model for Down syndrome. Prog. Clin. Biol. Res. 384, 117–133 (1993). - PubMed
-
- Reeves R. H., et al. , A mouse model for Down syndrome exhibits learning and behaviour deficits. Nat. Genet. 11, 177–184 (1995). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

