Preventing presbycusis in mice with enhanced medial olivocochlear feedback
- PMID: 32393641
- PMCID: PMC7261056
- DOI: 10.1073/pnas.2000760117
Preventing presbycusis in mice with enhanced medial olivocochlear feedback
Abstract
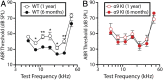
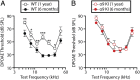
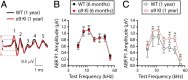
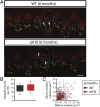
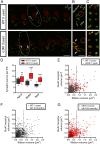
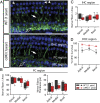
"Growing old" is the most common cause of hearing loss. Age-related hearing loss (ARHL) (presbycusis) first affects the ability to understand speech in background noise, even when auditory thresholds in quiet are normal. It has been suggested that cochlear denervation ("synaptopathy") is an early contributor to age-related auditory decline. In the present work, we characterized age-related cochlear synaptic degeneration and hair cell loss in mice with enhanced α9α10 cholinergic nicotinic receptors gating kinetics ("gain of function" nAChRs). These mediate inhibitory olivocochlear feedback through the activation of associated calcium-gated potassium channels. Cochlear function was assessed via distortion product otoacoustic emissions and auditory brainstem responses. Cochlear structure was characterized in immunolabeled organ of Corti whole mounts using confocal microscopy to quantify hair cells, auditory neurons, presynaptic ribbons, and postsynaptic glutamate receptors. Aged wild-type mice had elevated acoustic thresholds and synaptic loss. Afferent synapses were lost from inner hair cells throughout the aged cochlea, together with some loss of outer hair cells. In contrast, cochlear structure and function were preserved in aged mice with gain-of-function nAChRs that provide enhanced olivocochlear inhibition, suggesting that efferent feedback is important for long-term maintenance of inner ear function. Our work provides evidence that olivocochlear-mediated resistance to presbycusis-ARHL occurs via the α9α10 nAChR complexes on outer hair cells. Thus, enhancement of the medial olivocochlear system could be a viable strategy to prevent age-related hearing loss.
Keywords: aging; cochlear synaptopathy; hearing loss; medial olivocochlear system.
Conflict of interest statement
The authors declare no competing interest.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

