Pancreatic cancer stroma: an update on therapeutic targeting strategies
- PMID: 32393771
- PMCID: PMC8284850
- DOI: 10.1038/s41575-020-0300-1
Pancreatic cancer stroma: an update on therapeutic targeting strategies
Abstract
Pancreatic ductal adenocarcinoma (PDAC) is a leading cause of cancer-related mortality in the Western world with limited therapeutic options and dismal long-term survival. The neoplastic epithelium exists within a dense stroma, which is recognized as a critical mediator of disease progression through direct effects on cancer cells and indirect effects on the tumour immune microenvironment. The three dominant entities in the PDAC stroma are extracellular matrix (ECM), vasculature and cancer-associated fibroblasts (CAFs). The ECM can function as a barrier to effective drug delivery to PDAC cancer cells, and a multitude of strategies to target the ECM have been attempted in the past decade. The tumour vasculature is a complex system and, although multiple anti-angiogenesis agents have already failed late-stage clinical trials in PDAC, other vasculature-targeting approaches aimed at vessel normalization and tumour immunosensitization have shown promise in preclinical models. Lastly, PDAC CAFs participate in active cross-talk with cancer cells within the tumour microenvironment. The existence of intratumoural CAF heterogeneity represents a paradigm shift in PDAC CAF biology, with myofibroblastic and inflammatory CAF subtypes that likely make distinct contributions to PDAC progression. In this Review, we discuss our current understanding of the three principal constituents of PDAC stroma, their effect on the prevalent immune landscape and promising therapeutic targets within this compartment.
Conflict of interest statement
Competing interests
A.M. receives royalties from Cosmos Wisdom Biotechnology for a biomarker assay related to early detection of pancreatic cancer. A.M. is an inventor on a patent that has been licensed to Thrive Earlier Detection. The remaining authors declare no competing interests.
Figures




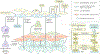
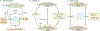
References
-
- American Cancer Society. Facts and Figures (American Cancer Society, 2019).
-
- Bray F et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin 68, 394–424 (2018). - PubMed
-
- Li D et al. Pancreatic cancer. Lancet 363, 1049–1057 (2004). - PubMed
-
- Conroy T et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med 364, 1817–1825 (2011). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

