A co-formulation of supramolecularly stabilized insulin and pramlintide enhances mealtime glucagon suppression in diabetic pigs
- PMID: 32393892
- PMCID: PMC7274092
- DOI: 10.1038/s41551-020-0555-4
A co-formulation of supramolecularly stabilized insulin and pramlintide enhances mealtime glucagon suppression in diabetic pigs
Abstract
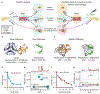
Treatment of patients with diabetes with insulin and pramlintide (an amylin analogue) is more effective than treatment with insulin only. However, because mixtures of insulin and pramlintide are unstable and have to be injected separately, amylin analogues are only used by 1.5% of people with diabetes needing rapid-acting insulin. Here, we show that the supramolecular modification of insulin and pramlintide with cucurbit[7]uril-conjugated polyethylene glycol improves the pharmacokinetics of the dual-hormone therapy and enhances postprandial glucagon suppression in diabetic pigs. The co-formulation is stable for over 100 h at 37 °C under continuous agitation, whereas commercial formulations of insulin analogues aggregate after 10 h under similar conditions. In diabetic rats, the administration of the stabilized co-formulation increased the area-of-overlap ratio of the pharmacokinetic curves of pramlintide and insulin from 0.4 ± 0.2 to 0.7 ± 0.1 (mean ± s.d.) for the separate administration of the hormones. The co-administration of supramolecularly stabilized insulin and pramlintide better mimics the endogenous kinetics of co-secreted insulin and amylin, and holds promise as a dual-hormone replacement therapy.
Conflict of interest statement
Competing interests
E.A.A., B.A.B., D.M.M., C.L.M., and G.A.R. are inventors on a patent filing describing the work reported in this manuscript.
Figures
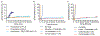

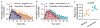


Comment in
-
Supramolecularly stabilized diabetes drugs.Nat Biomed Eng. 2020 May;4(5):481-482. doi: 10.1038/s41551-020-0558-1. Nat Biomed Eng. 2020. PMID: 32393893 No abstract available.
References
-
- WHO. Diabetes: Key Facts. World Health Organization; (2017).
-
- Borm AK et al. The effect of pramlintide (amylin analogue) treatment on bone metabolism and bone density in patients with type 1 diabetes mellitus. Horm. Metab. Res 31, 472–475 (1999). - PubMed
-
- Gottlieb A et al. Pramlintide as an adjunct to insulin therapy improved glycemic and weight control in people with type 1 diabetes during treatment for 52 weeks. Diabetes. 49, A109–A109 (2000).
-
- Ryan GJ, Jobe LJ & Martin R Pramlintide in the treatment of type 1 and type 2 diabetes mellitus. Clin. Ther 27, 1500–1512 (2005). - PubMed
-
- Edelman S et al. A double-blind, placebo-controlled trial assessing pramlintide treatment in the setting of intensive insulin therapy in type 1 diabetes. Diabetes Care. 29, 2189–2195 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

