Assessment of proliferation, migration and differentiation potentials of bone marrow mesenchymal stem cells labeling with silica-coated and amine-modified superparamagnetic iron oxide nanoparticles
- PMID: 32394163
- PMCID: PMC7450019
- DOI: 10.1007/s10616-020-00397-5
Assessment of proliferation, migration and differentiation potentials of bone marrow mesenchymal stem cells labeling with silica-coated and amine-modified superparamagnetic iron oxide nanoparticles
Abstract
Superparamagnetic iron oxide nanoparticles have been widely used for cell labeling in preclinical and clinical studies, to improve labeling efficiency, particle conjugation and surface modifications are developed, but some modified SPIONs exert side-effect on physiological activity of cells, which cannot be served as ideal cell tracker. In this study, amine-modified silica-coated SPIO (SPIO@SiO2-NH2, SPIO@S-N) nanoparticles were used to label bone marrow derived mesenchymal stem cells (BM-MSCs), then the stem cell potentials were evaluated. It was found BM-MSCs could be efficiently labeled by SPIO@S-N nanoparticles. After labeling, the BM-MSCs viability kept well and the migration ability increased, but the osteogenesis and adipogenesis potentials were not impaired. In steroid associated osteonecrosis (SAON) bone defect model, stem cell implantation was performed by injection of SPIO@S-N labeled BM-MSCs into marrow cavity locally, it was found the SPIO positive cells homed to the periphery of defect region in control group, but were recruited to the defect region in poly lactic-coglycolic acid/tricalcium phosphate (PLGA/TCP) scaffold implantation group. In conclusion, SPIO@S-N nanoparticles promoted migration while retained proliferation and differentiation ability of BM-MSCs, implying this kind of nanoparticles could be served not only an ideal tracking marker but also an accelerator for stem cell homing during tissue repair.
Keywords: Differentiation; Mesenchymal stem cells; Migration; Steroid associated osteonecrosis; Superparamagnetic iron oxide nanoparticles; Viability.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures
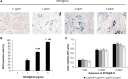


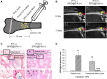
References
-
- Andreas K, Georgieva R, Ladwig M, Mueller S, Notter M, Sittinger M, Ringe J. Highly efficient magnetic stem cell labeling with citrate-coated superparamagnetic iron oxide nanoparticles for MRI tracking. Biomaterials. 2012;33:4515–4525. - PubMed
-
- Askari AT, et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. - PubMed
-
- Bulte JW, et al. Magnetodendrimers allow endosomal magnetic labeling and in vivo tracking of stem cells. Nat Biotechnol. 2001;19:1141–1147. - PubMed
-
- Chang YK, Liu YP, Ho JH, Hsu SC, Lee OK. Amine-surface-modified superparamagnetic iron oxide nanoparticles interfere with differentiation of human mesenchymal stem cells. J Orthop Res. 2012;30:1499–1506. - PubMed
-
- Chen YC, et al. The inhibitory effect of superparamagnetic iron oxide nanoparticle (Ferucarbotran) on osteogenic differentiation and its signaling mechanism in human mesenchymal stem cells. Toxicol Appl Pharmacol. 2010;245:272–279. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

