Glucose transporters in pancreatic islets
- PMID: 32394191
- PMCID: PMC7462922
- DOI: 10.1007/s00424-020-02383-4
Glucose transporters in pancreatic islets
Abstract
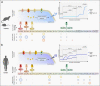
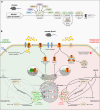
The fine-tuning of glucose uptake mechanisms is rendered by various glucose transporters with distinct transport characteristics. In the pancreatic islet, facilitative diffusion glucose transporters (GLUTs), and sodium-glucose cotransporters (SGLTs) contribute to glucose uptake and represent important components in the glucose-stimulated hormone release from endocrine cells, therefore playing a crucial role in blood glucose homeostasis. This review summarizes the current knowledge about cell type-specific expression profiles as well as proven and putative functions of distinct GLUT and SGLT family members in the human and rodent pancreatic islet and further discusses their possible involvement in onset and progression of diabetes mellitus. In context of GLUTs, we focus on GLUT2, characterizing the main glucose transporter in insulin-secreting β-cells in rodents. In addition, we discuss recent data proposing that other GLUT family members, namely GLUT1 and GLUT3, render this task in humans. Finally, we summarize latest information about SGLT1 and SGLT2 as representatives of the SGLT family that have been reported to be expressed predominantly in the α-cell population with a suggested functional role in the regulation of glucagon release.
Keywords: GLUTs; Glucose transport; Pancreatic islet; SGLTs; α-Cell; β-Cell.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

