Synthetic strategies, SAR studies, and computer modeling of indole 2 and 3-carboxamides as the strong enzyme inhibitors: a review
- PMID: 32394235
- PMCID: PMC7214098
- DOI: 10.1007/s11030-020-10061-x
Synthetic strategies, SAR studies, and computer modeling of indole 2 and 3-carboxamides as the strong enzyme inhibitors: a review
Abstract
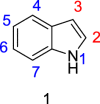
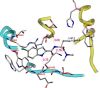
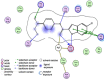
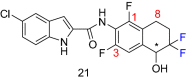
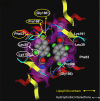
Indole derivatives have been the focus of many researchers in the study of pharmaceutical compounds for many years. Researchers have investigated the effect of carboxamide moiety at positions 2 and 3, giving unique inhibitory properties to these compounds. The presence of carboxamide moiety in indole derivatives causes hydrogen bonds with a variety of enzymes and proteins, which in many cases, inhibits their activity. In this review, synthetic strategies of indole 2 and 3-carboxamide derivatives, the type, and mode of interaction of these derivatives against HLGP, HIV-1, renin enzyme, and structure-activity studies of these compounds were investigated. It is hoped that indole scaffolds will be tested in the future for maximum activity in pharmacological compounds.
Keywords: Carboxamide moiety; HIV-1; HLGP; Indole; Inhibitory activity; Renin.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- Sundberg RJ. Chem of indoles. New York: Academic Press; 1996.
-
- Ciulla MG, Kumar K. The natural and synthetic indole weaponry against bacteria. Tetrahedron Lett. 2018;59(34):3223–3233. doi: 10.1016/j.tetlet.2018.07.045. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

