Devastating intimacy: the cell biology of plant-Phytophthora interactions
- PMID: 32394464
- PMCID: PMC7540312
- DOI: 10.1111/nph.16650
Devastating intimacy: the cell biology of plant-Phytophthora interactions
Abstract
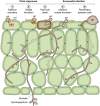
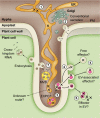
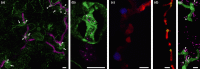
An understanding of the cell biology underlying the burgeoning molecular genetic and genomic knowledge of oomycete pathogenicity is essential to gain the full context of how these pathogens cause disease on plants. An intense research focus on secreted Phytophthora effector proteins, especially those containing a conserved N-terminal RXLR motif, has meant that most cell biological studies into Phytophthora diseases have focussed on the effectors and their host target proteins. While these effector studies have provided novel insights into effector secretion and host defence mechanisms, there remain many unanswered questions about fundamental processes involved in spore biology, host penetration and haustorium formation and function.
Keywords: Phytophthora; RXLR; effector; haustoria; plant defence; plant-pathogen interactions.
© 2020 The Authors New Phytologist © 2020 New Phytologist Trust.
Figures


References
-
- Aram K, Rizzo DM. 2018. Distinct trophic specializations affect how Phytophthora ramorum and Clade 6 Phytophthora spp. colonize and persist on Umbellularia californica leaves in streams. Phytopathology 108: 858–869. - PubMed
-
- Armstrong MR, Whisson SC, Pritchard L, Bos JIB, Venter E, Avrova AO, Rehmany AP, Böhme U, Brooks K, Cherevach I et al 2005. An ancestral oomycete locus contains late blight avirulence gene Avr3a, encoding a protein that is recognized in the host cytoplasm. Proceedings of the National Academy of Sciences, USA 102: 7766–7771. - PMC - PubMed
-
- Avrova AO, Boevink PC, Young V, Grenville‐Briggs LJ, van West P, Birch PRJ, Whisson SC. 2008. A novel Phytophthora infestans haustorium‐specific membrane protein is required for infection of potato. Cellular Microbiology 10: 2271–2284. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

