New developments in transcatheter therapy of mitral valve disease
- PMID: 32395315
- PMCID: PMC7212154
- DOI: 10.21037/jtd.2019.12.137
New developments in transcatheter therapy of mitral valve disease
Abstract
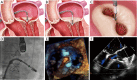
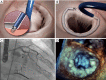
Mitral valve regurgitation (MR) belongs to one of the most common acquired valve diseases in western countries with increasing prevalence in older age. For patients with high perioperative risk and older age prohibitive for valve surgery, the development of transcatheter mitral valve therapies offers new options. Assessment of the severity and etiology of MR and thorough imaging of the mitral valve anatomy and pathology are necessary prerequisites for appropriate decision making in the field of transcatheter mitral valve therapies. Different transcatheter repair and replacement techniques are on the market, most of them mimicking surgical techniques. With some techniques (e.g., the MitraClip device), there is good clinical experience (>80,000 devices implanted worldwide), and evidence (three randomized studies), whereas for newer procedures, safety and efficacy data are still very limited. Transcatheter mitral repair and replacement techniques have to be considered as complementary treatment options for high-risk patients indicated by the Heart Teams. The different techniques and devices will be introduced and discussed in the following paper.
Keywords: MitraClip device; Mitral valve regurgitation (MR); degenerative mitral regurgitation; functional mitral regurgitation; heart failure; percutaneous mitral repair; transcatheter mitral valve repair.
2020 Journal of Thoracic Disease. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2019.12.137). The series “Interventional Cardiology” was commissioned by the editorial office without any funding or sponsorship. CW received honoraria from Abbot Vascular. TW received honoraria from Abbot Vascular and Edwards Lifesciences. SF received honoraria from Abbot Vascular and Edwards Lifesciences. Mariuca Vasa-Nicotera received honoraria from Abbot Vascular and Edwards Lifesciences. TH and MA have no other conflicts of interest.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
