miR-122/SIRT1 axis regulates chondrocyte extracellular matrix degradation in osteoarthritis
- PMID: 32395770
- PMCID: PMC7308613
- DOI: 10.1042/BSR20191908
miR-122/SIRT1 axis regulates chondrocyte extracellular matrix degradation in osteoarthritis
Abstract
Background/aims: MicroRNAs (miRNAs) are involved in the pathogenesis of osteoarthritis (OA). The present study aimed to investigate the potential function of miR-122 in the development of OA and its potential molecular mechanisms.
Methods: The expression of miR-122, silent information regulator 1 (SIRT1), collagen II, aggrecan, matrix metalloproteinase (MMP) 13 (MMP13) and ADAMTS4 in OA cartilage was detected by RT-qPCR. Target gene prediction and screening, luciferase reporter assay were used to verify downstream target genes of miR-122.
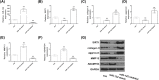
Results: Compared with osteonecrosis, the expression of miR-122 was significantly increased in OA cartilage, while the expression of SIRT1 was significantly decreased. Overexpression of miR-122 increased the expression of extracellular matrix (ECM) catabolic factors, for example disintegrins, MMPs and metalloproteinases with platelet reaction protein motifs, and inhibited the expression of synthetic metabolic genes such as collagen II and aggregating proteoglycan. Inhibition of miR-122 expression had the opposite effect. Furthermore, SIRT1 was identified as a direct target of miR-122. SIRT1 was significantly inhibited by miR-122 overexpression. Knockdown of SIRT1 reversed the degradation of chondrocyte ECM by miR-122 inhibitors.
Conclusion: The miR-122/SIRT1 axis can regulate the degradation of ECM in OA, thus providing new insights into the treatment of OA.
Keywords: ECM; SIRT1; chondrocytes; miR-122; osteoarthritis.
© 2020 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures
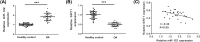


References
-
- Jevotovsky D.S., Alfonso A.R., Einhorn T.A. and Chiu E.S. (2018) Osteoarthritis and stem cell therapy in humans: a systematic review. Osteoarthritis Cartilage 26, 711. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

