Structural Determinants of Phosphopeptide Binding to the N-Terminal Src Homology 2 Domain of the SHP2 Phosphatase
- PMID: 32395997
- PMCID: PMC8007070
- DOI: 10.1021/acs.jcim.0c00307
Structural Determinants of Phosphopeptide Binding to the N-Terminal Src Homology 2 Domain of the SHP2 Phosphatase
Abstract
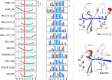
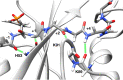
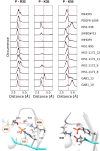
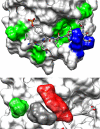
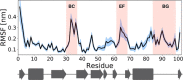
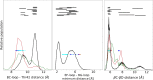
SH2 domain-containing tyrosine phosphatase 2 (SHP2), encoded by PTPN11, plays a fundamental role in the modulation of several signaling pathways. Germline and somatic mutations in PTPN11 are associated with different rare diseases and hematologic malignancies, and recent studies have individuated SHP2 as a central node in oncogenesis and cancer drug resistance. The SHP2 structure includes two Src homology 2 domains (N-SH2 and C-SH2) followed by a catalytic protein tyrosine phosphatase (PTP) domain. Under basal conditions, the N-SH2 domain blocks the active site, inhibiting phosphatase activity. Association of the N-SH2 domain with binding partners containing short amino acid motifs comprising a phosphotyrosine residue (pY) leads to N-SH2/PTP dissociation and SHP2 activation. Considering the relevance of SHP2 in signaling and disease and the central role of the N-SH2 domain in its allosteric regulation mechanism, we performed microsecond-long molecular dynamics (MD) simulations of the N-SH2 domain complexed to 12 different peptides to define the structural and dynamical features determining the binding affinity and specificity of the domain. Phosphopeptide residues at position -2 to +5, with respect to pY, have significant interactions with the SH2 domain. In addition to the strong interaction of the pY residue with its conserved binding pocket, the complex is stabilized hydrophobically by insertion of residues +1, +3, and +5 in an apolar groove of the domain and interaction of residue -2 with both the pY and a protein surface residue. Additional interactions are provided by hydrogen bonds formed by the backbone of residues -1, +1, +2, and +4. Finally, negatively charged residues at positions +2 and +4 are involved in electrostatic interactions with two lysines (Lys89 and Lys91) specific for the SHP2 N-SH2 domain. Interestingly, the MD simulations illustrated a previously undescribed conformational flexibility of the domain, involving the core β sheet and the loop that closes the pY binding pocket.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
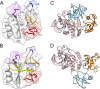


References
-
- Mayer B. J.What Have We Learned from SH2 Domains? In SH2 Domains; Liu B. A., Machida K., Eds.; Humana Press: New York, 2017; pp 37–43.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

