Deep-Learning Image Reconstruction for Real-Time Photoacoustic System
- PMID: 32396076
- PMCID: PMC8594135
- DOI: 10.1109/TMI.2020.2993835
Deep-Learning Image Reconstruction for Real-Time Photoacoustic System
Abstract
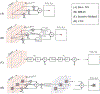
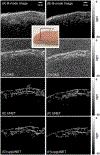
Recent advances in photoacoustic (PA) imaging have enabled detailed images of microvascular structure and quantitative measurement of blood oxygenation or perfusion. Standard reconstruction methods for PA imaging are based on solving an inverse problem using appropriate signal and system models. For handheld scanners, however, the ill-posed conditions of limited detection view and bandwidth yield low image contrast and severe structure loss in most instances. In this paper, we propose a practical reconstruction method based on a deep convolutional neural network (CNN) to overcome those problems. It is designed for real-time clinical applications and trained by large-scale synthetic data mimicking typical microvessel networks. Experimental results using synthetic and real datasets confirm that the deep-learning approach provides superior reconstructions compared to conventional methods.
Figures








References
-
- Szabo TL, Diagnostic ultrasound imaging: inside out. Academic Press, 2004.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

