Cenobamate (YKP3089) as adjunctive treatment for uncontrolled focal seizures in a large, phase 3, multicenter, open-label safety study
- PMID: 32396252
- PMCID: PMC7317552
- DOI: 10.1111/epi.16525
Cenobamate (YKP3089) as adjunctive treatment for uncontrolled focal seizures in a large, phase 3, multicenter, open-label safety study
Abstract
Objective: During the development of cenobamate, an antiseizure medication (ASM) for focal seizures, three cases of drug reaction with eosinophilia and systemic symptoms (DRESS) occurred. To mitigate the rate of DRESS, a start-low, go-slow approach was studied in an ongoing, open-label, multicenter study. Also examined were long-term safety of cenobamate and a method for managing the pharmacokinetic interaction between cenobamate, a 2C19 inhibitor, and concomitant phenytoin or phenobarbital.
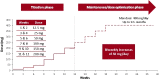
Methods: Patients 18-70 years old with uncontrolled focal seizures taking stable doses of one to three ASMs were enrolled. Cenobamate 12.5 mg/d was initiated and increased at 2-week intervals to 25, 50, 100, 150, and 200 mg/d. Additional biweekly 50 mg/d increases to 400 mg/d were allowed. During titration, patients taking phenytoin or phenobarbital could not have their cenobamate titration rate or other concomitant ASMs adjusted; phenytoin/phenobarbital doses could be decreased by 25%-33%.
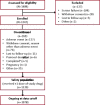
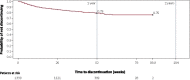
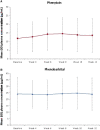
Results: At data cutoff (median treatment duration = 9 months), 1347 patients were enrolled, of whom 269 (20.0%) discontinued, most commonly due to adverse events (n = 137) and consent withdrawn for reason other than adverse event (n = 74); 1339 patients received ≥1 treatment dose (median modal dose = 200 mg). The most common treatment-emergent adverse events (TEAEs) were somnolence (28.1%), dizziness (23.6%), and fatigue (16.6%). Serious TEAEs occurred in 108 patients (8.1%), most commonly seizure (n = 14), epilepsy (n = 5), and pneumonia, fall, and dizziness (n = 4 each). No cases of DRESS were identified. In the phenytoin/phenobarbital groups, 43.4% (36/114) and 29.7% (11/51) of patients, respectively, had their doses decreased. At the end of titration, mean plasma phenytoin/phenobarbital levels were generally comparable to baseline.
Significance: No cases of DRESS were identified in 1339 patients exposed to cenobamate using a start-low (12.5 mg/d), go-slow titration approach. Cenobamate was generally well tolerated in the long term, with no new safety issues found. Phenytoin/phenobarbital dose reductions (25%-33%), when needed during cenobamate titration, maintained stable plasma levels.
Keywords: DRESS; antiepileptic drugs; cenobamate; refractory epilepsy; safety/tolerability.
© 2020 The Authors. Epilepsia published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
M.R.S. is a consultant/advisor for Medtronic (fee to institution); is a consultant for NeurologyLive; has received research support (to institution) from Eisai, Engage, Medtronic, Neurelis, Pfizer, SK Life Science, Inc., Takeda, UCB, and Xenon; and has been a speaker for Eisai. P.K. is a consultant for AbbVie, Alliance, Eisai, Engage, and UCB Pharma; has participated on advisory boards for Aquestive and SK Life Science, Inc.; and has been a speaker for Eisai, Sunovion, and UCB Pharma. He has received research support from Eisai and Lundbeck. S.A. has participated on advisory boards for Eisai and SK Life Science, Inc., and has been a speaker for Sunovion and Eisai. M.G. has received research support from Aquestive, Biogen, Eisai, Engage, SK Life Science, Inc., and Upsher‐Smith. J.J.H. is a consultant/advisor for Brain Sentinel, Takeda, and NCGH; investigator for clinical trials funded by Biogen, Brain Sentinel, the Epilepsy Study Consortium, Greenwich Biosciences, SK Life Science, Inc., and Takeda; and member of the board of directors (stock owner) for CortiCare. G.L.K. is a consultant/advisor for Adamas, Eisai, Otsuka, and Shire; and has received research support from Biogen, SK Life Science, Inc., UCB Pharma, and Upsher‐Smith. W.E.R. is currently a consultant/advisor for SK Life Science, Inc. and previously for Eisai; has received honoraria for speaking from Eisai, Greenwich Biosciences (GW Pharmaceuticals), Sunovion, and UCB Pharma; and has received grant/research support from Greenwich Biosciences, Marinus, Medtronic, Neurelis, Ovid, SK Life Science, Inc., Takeda, UCB Pharma, and Upsher‐Smith. D.G.V. has received research support (to institution) from Biogen, Eisai, SK Life Science, Inc., and UCB Pharma; has participated on advisory boards for Otsuka and SK Life Science, Inc.; and has been a speaker for Eisai, Greenwich Biosciences, Lundbeck, Sunovion, and UCB Pharma. R.W. has been an advisor/consultant for Brain Sentinel, Eisai, Engage, Greenwich Biosciences, Lundbeck, SK Life Science, Inc., Sunovion, and UCB Pharma; has been a clinical investigator for Aquestive, Biogen, Eisai, Engage, Greenwich Biosciences; Lundbeck, Pfizer, SK Life Science, Inc., Sunovion, UCB Pharma, Xenon, and Zogenix; and has been a speaker for Aquestive, Eisai, Greenwich Biosciences, LivaNova, Sunovion, and UCB Pharma. L.B. and M.K. are employees of SK Life Science, Inc. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Figures
References
-
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342(5):314–9. - PubMed
-
- Kwan P, Brodie MJ. Effectiveness of first antiepileptic drug. Epilepsia. 2001;42(10):1255–60. - PubMed
-
- Giussani G, Bianchi E, Canelli V, et al. Antiepileptic drug discontinuation by people with epilepsy in the general population. Epilepsia. 2017;58(9):1524–32. - PubMed
-
- French JA, White HS, Klitgaard H, et al. Development of new treatment approaches for epilepsy: unmet needs and opportunities. Epilepsia. 2013;54(Suppl 4):3–12. - PubMed
-
- Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS. Progress report on new antiepileptic drugs: a summary of the Eleventh Eilat Conference (EILAT XI). Epilepsy Res. 2013;103(1):2–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

