The Evolution of Insulin and How it Informs Therapy and Treatment Choices
- PMID: 32396624
- PMCID: PMC7366348
- DOI: 10.1210/endrev/bnaa015
The Evolution of Insulin and How it Informs Therapy and Treatment Choices
Abstract
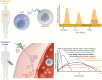
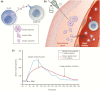
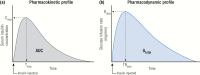
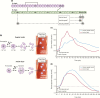
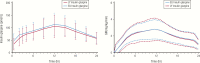
Insulin has been available for the treatment of diabetes for almost a century, and the variety of insulin choices today represents many years of discovery and innovation. Insulin has gone from poorly defined extracts of animal pancreata to pure and precisely controlled formulations that can be prescribed and administered with high accuracy and predictability of action. Modifications of the insulin formulation and of the insulin molecule itself have made it possible to approximate the natural endogenous insulin response. Insulin and insulin formulations had to be designed to produce either a constant low basal level of insulin or the spikes of insulin released in response to meals. We discuss how the biochemical properties of endogenous insulin were exploited to either shorten or extend the time-action profiles of injectable insulins by varying the pharmacokinetics (time for appearance of insulin in the blood after injection) and pharmacodynamics (time-dependent changes in blood sugar after injection). This has resulted in rapid-acting, short-acting, intermediate-acting, and long-acting insulins, as well as mixtures and concentrated formulations. An understanding of how various insulins and formulations were designed to solve the challenges of insulin replacement will assist clinicians in meeting the needs of their individual patients.
Keywords: insulin; long-acting; pharmacodynamics; pharmacokinetics; rapid-acting.
© Endocrine Society 2020.
Figures




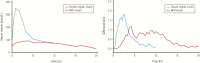

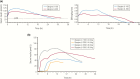





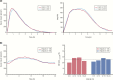
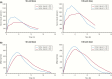
References
-
- Bliss M. The Discovery of Insulin. Chicago, IL: The University of Chicago Press: 1982.
-
- Nicol DS, Smith LF. Amino-acid sequence of human insulin. Nature. 1960;187:483–485. - PubMed
-
- Galloway JA, Hooper SA, Spradlin CT, et al. Biosynthetic human proinsulin. Review of chemistry, in vitro and in vivo receptor binding, animal and human pharmacology studies, and clinical trial experience. Diabetes Care. 1992;15(5):666–692. - PubMed
-
- Dunn MF. Zinc-ligand interactions modulate assembly and stability of the insulin hexamer – a review. Biometals. 2005;18(4):295–303. - PubMed
-
- Owens DR, Jones MK, Birtwell AJ, et al. Pharmacokinetics of subcutaneously administered human, porcine and bovine neutral soluble insulin to normal man. Horm Metab Res. 1984;16 Suppl 1:195–199. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

