Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19
- PMID: 32396996
- PMCID: PMC7272948
- DOI: 10.1111/all.14364
Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19
Abstract
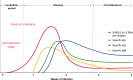
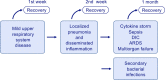
As a zoonotic disease that has already spread globally to several million human beings and possibly to domestic and wild animals, eradication of coronavirus disease 2019 (COVID-19) appears practically impossible. There is a pressing need to improve our understanding of the immunology of this disease to contain the pandemic by developing vaccines and medicines for the prevention and treatment of patients. In this review, we aim to improve our understanding on the immune response and immunopathological changes in patients linked to deteriorating clinical conditions such as cytokine storm, acute respiratory distress syndrome, autopsy findings and changes in acute-phase reactants, and serum biochemistry in COVID-19. Similar to many other viral infections, asymptomatic disease is present in a significant but currently unknown fraction of the affected individuals. In the majority of the patients, a 1-week, self-limiting viral respiratory disease typically occurs, which ends with the development of neutralizing antiviral T cell and antibody immunity. The IgM-, IgA-, and IgG-type virus-specific antibodies levels are important measurements to predict population immunity against this disease and whether cross-reactivity with other coronaviruses is taking place. High viral load during the first infection and repeated exposure to virus especially in healthcare workers can be an important factor for severity of disease. It should be noted that many aspects of severe patients are unique to COVID-19 and are rarely observed in other respiratory viral infections, such as severe lymphopenia and eosinopenia, extensive pneumonia and lung tissue damage, a cytokine storm leading to acute respiratory distress syndrome, and multiorgan failure. Lymphopenia causes a defect in antiviral and immune regulatory immunity. At the same time, a cytokine storm starts with extensive activation of cytokine-secreting cells with innate and adaptive immune mechanisms both of which contribute to a poor prognosis. Elevated levels of acute-phase reactants and lymphopenia are early predictors of high disease severity. Prevention of development to severe disease, cytokine storm, acute respiratory distress syndrome, and novel approaches to prevent their development will be main routes for future research areas. As we learn to live amidst the virus, understanding the immunology of the disease can assist in containing the pandemic and in developing vaccines and medicines to prevent and treat individual patients.
Keywords: COVID-19; cytokine storm; immune response; immunologic tests; immunopathology; infections; pandemic; virus.
© 2020 EAACI and John Wiley and Sons A/S. Published by John Wiley and Sons Ltd.
Conflict of interest statement
LM reports personal fees from AHL and grants from GSK. KN reports grants and/or personal fees and/or other from NIAID, Novartis, Regeneron, FARE, EAT, Sanofi, Astellas, Nestle, BeforeBrands, Alladapt, ForTra, Genentech, AImmune Therapeutics, DBV Technologies, AstraZeneca, ImmuneWorks, Cour Pharmaceuticals, AllerGenis, Ukko Pharma, AnaptysBio, Adare Pharmaceuticals, Stallergenes‐Greer, NHLBI, NIEHS, EPA, WAO Center of Excellence, Iggenix, Probio, Vedanta, Centocor, Seed, Immune Tolerance Network, and NIH. In addition, KN has pending patents on Inhibition of Allergic Reaction to Peanut Allergen using an IL‐33 Inhibitor, Special Oral Formula for Decreasing Food Allergy Risk and Treatment for Food Allergy, Basophil Activation Based Diagnostic Allergy Test, Granulocyte‐based methods for detecting and monitoring immune system disorders, Methods and Assays for Detecting and Quantifying Pure Subpopulations of White Blood Cells in Immune System Disorders, Mixed Allergen Compositions and Methods for Using the Same, and Microfluidic Device and Diagnostic Methods for Allergy Testing Based on Detection of Basophil Activation. CA reports grants from Allergopharma, Idorsia, Swiss National Science Foundation, Christine Kühne‐Center for Allergy Research and Education, European Commission's Horizon's 2020 Framework Programme, Cure, Novartis Research Institutes, AstraZeneca, SciBase, and advisory role in Sanofi/Regeneron. All other authors have no conflict of interest within the scope of the submitted work.
Figures







References
-
- Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814‐1820. - PubMed
-
- WHO . Coronavirus disease (COVID‐2019) situation reports. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situatio.... Accessed April 9
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

