Stimuli-Responsive Hydrogels for Local Post-Surgical Drug Delivery
- PMID: 32397180
- PMCID: PMC7345431
- DOI: 10.3390/gels6020014
Stimuli-Responsive Hydrogels for Local Post-Surgical Drug Delivery
Abstract
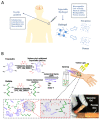
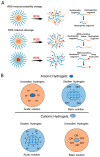
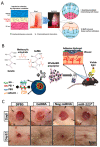
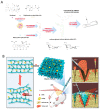
Currently, surgical operations, followed by systemic drug delivery, are the prevailing treatment modality for most diseases, including cancers and trauma-based injuries. Although effective to some extent, the side effects of surgery include inflammation, pain, a lower rate of tissue regeneration, disease recurrence, and the non-specific toxicity of chemotherapies, which remain significant clinical challenges. The localized delivery of therapeutics has recently emerged as an alternative to systemic therapy, which not only allows the delivery of higher doses of therapeutic agents to the surgical site, but also enables overcoming post-surgical complications, such as infections, inflammations, and pain. Due to the limitations of the current drug delivery systems, and an increasing clinical need for disease-specific drug release systems, hydrogels have attracted considerable interest, due to their unique properties, including a high capacity for drug loading, as well as a sustained release profile. Hydrogels can be used as local drug performance carriers as a means for diminishing the side effects of current systemic drug delivery methods and are suitable for the majority of surgery-based injuries. This work summarizes recent advances in hydrogel-based drug delivery systems (DDSs), including formulations such as implantable, injectable, and sprayable hydrogels, with a particular emphasis on stimuli-responsive materials. Moreover, clinical applications and future opportunities for this type of post-surgery treatment are also highlighted.
Keywords: drug delivery systems; hydrogel; implantable; injectable; sprayable.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Veronesi U., Luini A., Del Vecchio M., Greco M., Galimberti V., Merson M., Rilke F., Sacchini V., Saccozzi R., Savio T., et al. Radiotherapy after Breast-Preserving Surgery in Women with Localized Cancer of the Breast. N. Engl. J. Med. 1993;328:1587–1591. doi: 10.1056/NEJM199306033282202. - DOI - PubMed
-
- Regine W., Winter K.A., Abrams R., Safran H., Hoffman J.P., Konski A., Benson A.B., Macdonald J.S., Rich T.A., Willett C.G. Fluorouracil-based chemoradiation with either gemcitabine or fluorouracil chemotherapy after resection of pancreatic adenocarcinoma: 5-year analysis of the U.S. Intergroup/RTOG 9704 phase III trial. Ann. Surg. Oncol. 2011;18:1319–1326. doi: 10.1245/s10434-011-1630-6. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

