The Effects of Pre-Existing Antibodies on Live-Attenuated Viral Vaccines
- PMID: 32397218
- PMCID: PMC7290594
- DOI: 10.3390/v12050520
The Effects of Pre-Existing Antibodies on Live-Attenuated Viral Vaccines
Abstract
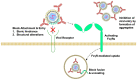
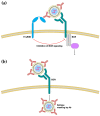
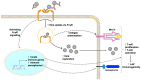
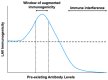
Live-attenuated vaccines (LAVs) have achieved remarkable successes in controlling virus spread, as well as for other applications such as cancer immunotherapy. However, with rapid increases in international travel, globalization, geographic spread of viral vectors, and widespread use of vaccines, there is an increasing need to consider how pre-exposure to viruses which share similar antigenic regions can impact vaccine efficacy. Pre-existing antibodies, derived from either from maternal-fetal transmission, or by previous infection or vaccination, have been demonstrated to interfere with vaccine immunogenicity of measles, adenovirus, and influenza LAVs. Immune interference of LAVs can be caused by the formation of virus-antibody complexes that neutralize virus infection in antigen-presenting cells, or by the cross-linking of the B-cell receptor with the inhibitory receptor, FcgRIIB. On the other hand, pre-existing antibodies can augment flaviviral LAV efficacy such as that of dengue and yellow fever virus, especially when pre-existing antibodies are present at sub-neutralizing levels. The increased vaccine immunogenicity can be facilitated by antibody-dependent enhancement of virus infection, enhancing virus uptake in antigen-presenting cells, and robust induction of innate immune responses that promote vaccine immunogenicity. This review examines the literature on this topic and examines the circumstances where pre-existing antibodies can inhibit or enhance LAV efficacy. A better knowledge of the underlying mechanisms involved could allow us to better manage immunization in seropositive individuals and even identify possibilities that could allow us to exploit pre-existing antibodies to boost vaccine-induced responses for improved vaccine efficacy.
Keywords: antibody-dependent enhancement; live-attenuated vaccine; pre-existing antibodies; vaccine immune interference; vaccine immunogenicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
African horse sickness virus (AHSV) with a deletion of 77 amino acids in NS3/NS3a protein is not virulent and a safe promising AHS Disabled Infectious Single Animal (DISA) vaccine platform.Vaccine. 2018 Apr 5;36(15):1925-1933. doi: 10.1016/j.vaccine.2018.03.003. Epub 2018 Mar 7. Vaccine. 2018. PMID: 29525278
-
Stable and Highly Immunogenic MicroRNA-Targeted Single-Dose Live Attenuated Vaccine Candidate against Tick-Borne Encephalitis Constructed Using Genetic Backbone of Langat Virus.mBio. 2019 Apr 23;10(2):e02904-18. doi: 10.1128/mBio.02904-18. mBio. 2019. PMID: 31015334 Free PMC article.
-
Dengue and Zika Virus Domain III-Flagellin Fusion and Glycan-Masking E Antigen for Prime-Boost Immunization.Theranostics. 2019 Jul 9;9(16):4811-4826. doi: 10.7150/thno.35919. eCollection 2019. Theranostics. 2019. PMID: 31367259 Free PMC article.
-
The Molecular Specificity of the Human Antibody Response to Dengue Virus Infections.Adv Exp Med Biol. 2018;1062:63-76. doi: 10.1007/978-981-10-8727-1_5. Adv Exp Med Biol. 2018. PMID: 29845525 Review.
-
Measles-derived vaccines to prevent emerging viral diseases.Microbes Infect. 2018 Oct-Nov;20(9-10):493-500. doi: 10.1016/j.micinf.2018.01.005. Epub 2018 Feb 1. Microbes Infect. 2018. PMID: 29410084 Free PMC article. Review.
Cited by
-
Neutralizing Antibodies and Antibody-Dependent Enhancement in COVID-19: A Perspective.J Indian Inst Sci. 2022;102(2):671-687. doi: 10.1007/s41745-021-00268-8. Epub 2022 Feb 4. J Indian Inst Sci. 2022. PMID: 35136306 Free PMC article. Review.
-
Respiratory Syncytial Virus Vaccines: A Review of the Candidates and the Approved Vaccines.Pathogens. 2023 Oct 19;12(10):1259. doi: 10.3390/pathogens12101259. Pathogens. 2023. PMID: 37887775 Free PMC article. Review.
-
Potentiating Lung Mucosal Immunity Through Intranasal Vaccination.Front Immunol. 2021 Dec 14;12:808527. doi: 10.3389/fimmu.2021.808527. eCollection 2021. Front Immunol. 2021. PMID: 34970279 Free PMC article. Review.
-
Universally Immune: How Infection Permissive Next Generation Influenza Vaccines May Affect Population Immunity and Viral Spread.Viruses. 2021 Sep 6;13(9):1779. doi: 10.3390/v13091779. Viruses. 2021. PMID: 34578360 Free PMC article. Review.
-
Impact of Pre-Existing Immunity to Influenza on Live-Attenuated Influenza Vaccine (LAIV) Immunogenicity.Vaccines (Basel). 2020 Nov 16;8(4):683. doi: 10.3390/vaccines8040683. Vaccines (Basel). 2020. PMID: 33207559 Free PMC article.
References
-
- Bristol N. William H Stewart. The Lancet. 2008;372:110. doi: 10.1016/S0140-6736(08)61022-3. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

