Effect of Antioxidants on High-Temperature Stability of Renewable Bio-Oils Revealed by an Innovative Method for the Determination of Kinetic Parameters of Oxidative Reactions
- PMID: 32397271
- PMCID: PMC7278824
- DOI: 10.3390/antiox9050399
Effect of Antioxidants on High-Temperature Stability of Renewable Bio-Oils Revealed by an Innovative Method for the Determination of Kinetic Parameters of Oxidative Reactions
Abstract
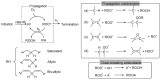
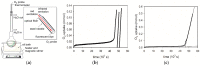
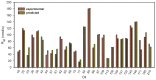
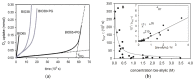
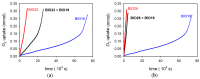
Bio-oils employed for various industrial purposes, such as biodiesel production, undergo extensive oxidation and degradation during transformation processes. Therefore, it is extremely important to predict their stability at high temperature. We report herein a new procedure based on the optically detected profile of headspace O2 concentration during isotherms at 130 °C for evaluating the oxidation kinetic parameters of several bio-oil feedstocks. The slope of O2 consumption and the induction period duration were related to the oil characteristics (molecular structure, acidity, and presence of intrinsic antioxidants or metals). The increase of the induction time caused by a standardized propyl gallate addition yielded a semiquantitative value of radical generation rate. Investigated oils included used cooking oils; mono-, di-, and triglycerides from natural sources; free fatty acids; transesterified oils; and their blends. With respect to other methods, this characterization presents the advantage of disentangling and evaluating the role of both fatty acids composition and naturally occurring antioxidants, and allows the development of rational strategies for antioxidant protection of oils and of their blends.
Keywords: antioxidant; biodiesel; induction period; kinetics; oil mixtures; oxidative stability; oxygen consumption; propyl gallate; radicals.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Singh D., Sharma D., Soni S.L., Sharma S., Sharma P.K., Jhalani A. A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel. 2020;262:116553. doi: 10.1016/j.fuel.2019.116553. - DOI
-
- Li X., Luo X., Jin Y., Li J., Zhang H., Zhang A., Xie J. Heterogeneous sulfur-free hydrodeoxygenation catalysts for selectively upgrading the renewable bio-oils to second generation biofuels. Renew. Sustain. Energy Rev. 2018;82:3762–3797. doi: 10.1016/j.rser.2017.10.091. - DOI
-
- Xin J., Imahara H., Saka S. Kinetics on the oxidation of biodiesel stabilized with antioxidant. Fuel. 2009;88:282–286. doi: 10.1016/j.fuel.2008.08.018. - DOI
LinkOut - more resources
Full Text Sources

