A Synergistic Effect of Reactive Oxygen and Reactive Nitrogen Species in Plasma Activated Liquid Media Triggers Astrocyte Wound Healing
- PMID: 32397300
- PMCID: PMC7247562
- DOI: 10.3390/ijms21093343
A Synergistic Effect of Reactive Oxygen and Reactive Nitrogen Species in Plasma Activated Liquid Media Triggers Astrocyte Wound Healing
Abstract
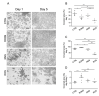
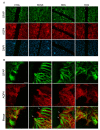
Astrocyte proliferation and migration toward injured Central Nervous System (CNS) areas are key features of astrogliosis and glial scar formation. Even though it is known that intracellular and environmental Reactive Oxygen and Nitrogen Species (RONS) affect astrocyte behaviour in physiological and pathophysiological conditions, their effects on the migration and growth of astrocytes are still unclear. Plasma-technologies are emerging in medicine as a tool to generate RONS for treating cells directly or through Plasma Activated Liquid Media (PALM). In this paper, we show for the first time how the use of PALM can modulate both astrocyte growth and migration as a function of active species produced by plasma in liquids. Our results show that PALM, generated by means of cold atmospheric pressure plasmas fed with N2, air or O2, can modulate astrocyte behaviour depending on the content of hydrogen peroxide and nitrite in the liquid. In particular, H2O2 enriched PALM induced a negative effect on cell growth associated with the mild wound healing improvement of primary astrocytes, in a scratch assay. Nitrite enriched PALM induced a selective effect on the wound healing without affecting cell growth. PALM containing a more balanced level of H2O2 and NO2- were able to affect cell growth, as well as significantly ameliorate wound healing. None of the PALM investigated induced upregulation of the gliotic inflammatory marker glial fibrillary acidic protein (GFAP), or of the astrocyte markers Aquaporin-4 (AQP4) and Connexin-43 (Cx-43) analysed by Western blot. Finally, immunofluorescence analysis revealed the presence of NO2- able to induce elongated protrusions at the front end of wounded astrocytes in the direction of cell migration. With our study we believe to have shown that PALM offer a novel tool to modulate astrocyte behaviour and that they are promising candidates for controlling astrogliosis in the case of CNS injuries.
Keywords: astrocytes; oxidative stress; plasma activated medium; wound healing.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

