Endoplasmic Reticulum Calcium Pumps and Tumor Cell Differentiation
- PMID: 32397400
- PMCID: PMC7247589
- DOI: 10.3390/ijms21093351
Endoplasmic Reticulum Calcium Pumps and Tumor Cell Differentiation
Abstract
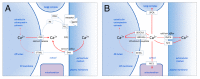
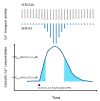
Endoplasmic reticulum (ER) calcium homeostasis plays an essential role in cellular calcium signaling, intra-ER protein chaperoning and maturation, as well as in the interaction of the ER with other organelles. Calcium is accumulated in the ER by sarco/endoplasmic reticulum calcium ATPases (SERCA enzymes) that generate by active, ATP-dependent transport, a several thousand-fold calcium ion concentration gradient between the cytosol (low nanomolar) and the ER lumen (high micromolar). SERCA enzymes are coded by three genes that by alternative splicing give rise to several isoforms, which can display isoform-specific calcium transport characteristics. SERCA expression levels and isoenzyme composition vary according to cell type, and this constitutes a mechanism whereby ER calcium homeostasis is adapted to the signaling and metabolic needs of the cell, depending on its phenotype, its state of activation and differentiation. As reviewed here, in several normal epithelial cell types including bronchial, mammary, gastric, colonic and choroid plexus epithelium, as well as in mature cells of hematopoietic origin such as pumps are simultaneously expressed, whereas in corresponding tumors and leukemias SERCA3 expression is selectively down-regulated. SERCA3 expression is restored during the pharmacologically induced differentiation of various cancer and leukemia cell types. SERCA3 is a useful marker for the study of cell differentiation, and the loss of SERCA3 expression constitutes a previously unrecognized example of the remodeling of calcium homeostasis in tumors.
Keywords: SERCA; calcium signaling; cancer; differentiation; endoplasmic reticulum; ion transport; leukemia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Lopez J.J., Jardin I., Albarrán L., Sanchez-Collado J., Cantonero C., Salido G.M., Smani T., Rosado J.A. Advances in Experimental Medicine and Biology. Vol. 1131. Springer; Berlin/Heidelberg, Germany: 2020. Molecular Basis and Regulation of Store-Operated Calcium Entry; pp. 445–469. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

