Hinge and Transmembrane Domains of Chimeric Antigen Receptor Regulate Receptor Expression and Signaling Threshold
- PMID: 32397414
- PMCID: PMC7291079
- DOI: 10.3390/cells9051182
Hinge and Transmembrane Domains of Chimeric Antigen Receptor Regulate Receptor Expression and Signaling Threshold
Abstract
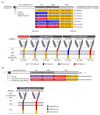
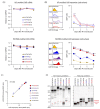
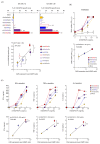
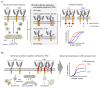
Chimeric antigen receptor (CAR)-T cells have demonstrated significant clinical potential; however, their strong antitumor activity may cause severe adverse effects. To ensure efficacy and safe CAR-T cell therapy, it is important to understand CAR's structure-activity relationship. To clarify the role of hinge and transmembrane domains in CAR and CAR-T cell function, we generated different chimeras and analyzed their expression levels and antigen-specific activity on CAR-T cells. First, we created a basic CAR with hinge, transmembrane, and signal transduction domains derived from CD3ζ, then we generated six CAR variants whose hinge or hinge/transmembrane domains originated from CD4, CD8α, and CD28. CAR expression level and stability on the T cell were greatly affected by transmembrane rather than hinge domain. Antigen-specific functions of most CAR-T cells depended on their CAR expression levels. However, CARs with a CD8α- or CD28-derived hinge domain showed significant differences in CAR-T cell function, despite their equal expression levels. These results suggest that CAR signaling intensity into T cells was affected not only by CAR expression level, but also by the hinge domain. Our discoveries indicate that the hinge domain regulates the CAR signaling threshold and the transmembrane domain regulates the amount of CAR signaling via control of CAR expression level.
Keywords: CAR-T cell therapy; chimeric antigen receptor; hinge domain; structure-activity relationship; transmembrane domain.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Tran E., Turcotte S., Gros A., Robbins P.F., Lu Y.C., Dudley M.E., Wunderlich J.R., Somerville R.P., Hogan K., Hinrichs C.S., et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344:641–645. doi: 10.1126/science.1251102. - DOI - PMC - PubMed
-
- Eshhar Z., Waks T., Gross G., Schindler D.G. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc. Natl. Acad. Sci. USA. 1993;90:720–724. doi: 10.1073/pnas.90.2.720. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

