Modulation of Lipoteichoic Acids and Exopolysaccharides Prevents Streptococcus mutans Biofilm Accumulation
- PMID: 32397430
- PMCID: PMC7249192
- DOI: 10.3390/molecules25092232
Modulation of Lipoteichoic Acids and Exopolysaccharides Prevents Streptococcus mutans Biofilm Accumulation
Abstract
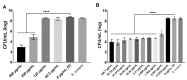
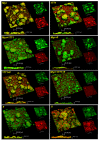
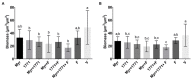
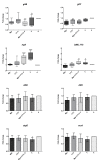
Dental caries is a diet-biofilm-dependent disease. Streptococcus mutans contributes to cariogenic biofilms by producing an extracellular matrix rich in exopolysaccharides and acids. The study aimed to determine the effect of topical treatments with compound 1771 (modulates lipoteichoic acid (LTA) metabolism) and myricetin (affects the synthesis of exopolysaccharides) on S. mutans biofilms. In vitro S. mutans UA159 biofilms were grown on saliva-coated hydroxyapatite discs, alternating 0.1% sucrose and 0.5% sucrose plus 1% starch. Twice-daily topical treatments were performed with both agents alone and combined with and without fluoride: compound 1771 (2.6 µg/mL), myricetin (500 µg/mL), 1771 + myricetin, fluoride (250 ppm), 1771 + fluoride, myricetin + fluoride, 1771 + myricetin + fluoride, and vehicle. Biofilms were evaluated via microbiological, biochemical, imaging, and gene expression methods. Compound 1771 alone yielded less viable counts, biomass, exopolysaccharides, and extracellular LTA. Moreover, the combination 1771 + myricetin + fluoride decreased three logs of bacterium counts, 60% biomass, >74% exopolysaccharides, and 20% LTA. The effect of treatments on extracellular DNA was not pronounced. The combination strategy affected the size of microcolonies and exopolysaccharides distribution and inhibited the expression of genes linked to insoluble exopolysaccharides synthesis. Therefore, compound 1771 prevented the accumulation of S. mutans biofilm; however, the effect was more pronounced when it was associated with fluoride and myricetin.
Keywords: Streptococcus mutans; biofilm; compound 1771; exopolysaccharides; lipoteichoic acids; myricetin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



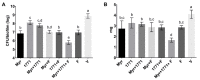



References
-
- Kassebaum N.J., Smith A.G.C., Bernabé E., Fleming T.D., Reynolds A.E., Vos T., Marcenes W. GBD 2015 Oral Health Collaborators. Global, Regional, and National Prevalence, Incidence, and Disability-Adjusted Life Years for Oral Conditions for 195 Countries, 1990–2015: A Systematic Analysis for the Global Burden of Diseases, Injuries, and Risk Factors. J. Dent. Res. 2017;96:380–387. doi: 10.1177/0022034517693566. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

