Synthesis and Therapeutic Applications of Iminosugars in Cystic Fibrosis
- PMID: 32397443
- PMCID: PMC7247015
- DOI: 10.3390/ijms21093353
Synthesis and Therapeutic Applications of Iminosugars in Cystic Fibrosis
Abstract
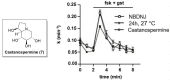
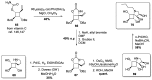
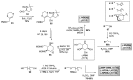
Iminosugars are sugar analogues endowed with a high pharmacological potential. The wide range of biological activities exhibited by these glycomimetics associated with their excellent drug profile make them attractive therapeutic candidates for several medical interventions. The ability of iminosugars to act as inhibitors or enhancers of carbohydrate-processing enzymes suggests their potential use as therapeutics for the treatment of cystic fibrosis (CF). Herein we review the most relevant advances in the field, paying attention to both the chemical synthesis of the iminosugars and their biological evaluations, resulting from in vitro and in vivo assays. Starting from the example of the marketed drug NBDNJ (N-butyl deoxynojirimycin), a variety of iminosugars have exhibited the capacity to rescue the trafficking of F508del-CFTR (deletion of F508 residue in the CF transmembrane conductance regulator), either alone or in combination with other correctors. Interesting results have also been obtained when iminosugars were considered as anti-inflammatory agents in CF lung disease. The data herein reported demonstrate that iminosugars hold considerable potential to be applied for both therapeutic purposes.
Keywords: CFTR correctors; anti-inflammatory agents; cystic fibrosis; cystic fibrosis transmembrane conductance regulator (CFTR); glycomimetics; glycosidase inhibitors; iminosugars; miglustat.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





















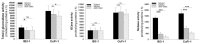


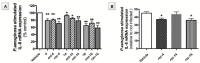

References
-
- Compain P., Martin O.R., editors. Iminosugars: From Synthesis to Therapeutic Applications. John Wiley & Sons, Ltd.; Chichester, UK: 2007.
-
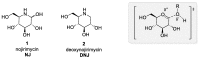
- Inouye S., Tsuruoka T., Nida T. The structure of nojirimycin, a piperidinose sugar antibiotic. J. Antibiot. (Tokyo) 1966;19:288–292. - PubMed
-
- Gao K., Zheng C., Wang T., Zhao H., Wang J., Wang Z., Zhai X., Jia Z., Chen J., Zhou Y., et al. 1-Deoxynojirimycin: Occurrence, Extraction, Chemistry, Oral Pharmacokinetics, Biological Activities and In Silico Target Fishing. Molecules. 2016;21:1600. doi: 10.3390/molecules21111600. - DOI - PMC - PubMed
-
- Stiitz A.E., editor. Iminosugars as Glycosidase Inhibitors. Wiley; Hoboken, NJ, USA: 1998.
-
- Asano N., Nash R.J., Molyneux R.J., Fleet G.W.J. Sugar-mimic glycosidase inhibitors: Natural occurrence, biological activity and prospects for therapeutic application. Tetrahedron Asymmetry. 2000;11:1645–1680. doi: 10.1016/S0957-4166(00)00113-0. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

