Toxicological Profile of Nanostructured Bone Substitute Based on Hydroxyapatite and Poly(lactide-co-glycolide) after Subchronic Oral Exposure of Rats
- PMID: 32397466
- PMCID: PMC7279500
- DOI: 10.3390/nano10050918
Toxicological Profile of Nanostructured Bone Substitute Based on Hydroxyapatite and Poly(lactide-co-glycolide) after Subchronic Oral Exposure of Rats
Abstract
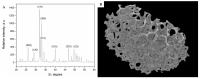
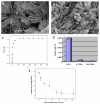
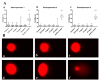
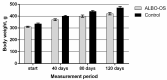
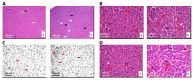
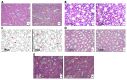
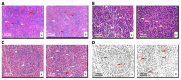
Novel three-dimensional (3D) nanohydroxyapatite-PLGA scaffolds with high porosity was developed to better mimic mineral component and microstructure of natural bone. To perform a final assessment of this nanomaterial as a potential bone substitute, its toxicological profile was particularly investigated. Therefore, we performed a comet assay on human monocytes for in vitro genotoxicity investigation, and the systemic subchronic toxicity investigation on rats being per oral feed with exactly administrated extract quantities of the nano calcium hydroxyapatite covered with tiny layers of PLGA (ALBO-OS) for 120 days. Histological and stereological parameters of the liver, kidney, and spleen tissue were analyzed. Comet assay revealed low genotoxic potential, while histological analysis and stereological investigation revealed no significant changes in exposed animals when compared to controls, although the volume density of blood sinusoids and connective tissue, as well as numerical density and number of mitosis were slightly increased. Additionally, despite the significantly increased average number of the Ki67 and slightly increased number of CD68 positive cells in the presence of ALBO-OS, immunoreactive cells proliferation was almost neglected. Blood analyses showed that all of the blood parameters in rats fed with extract nanomaterial are comparable with corresponding parameters of no feed rats, taken as blind probe. This study contributes to the toxicological profiling of ALBO-OS scaffold for potential future application in bone tissue engineering.
Keywords: biocompatibility; bone substitute; genotoxicity; hydroxyapatite; subchronic toxicity.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
-
- Tohamy K.M., Mabrouk M., Soliman I.E., Beherei H.H., Aboelnasr M.A. Novel alginate/hydroxyethyl cellulose/hydroxyapatite composite scaffold for bone regeneration: In Vitro cell viability and proliferation of human mesenchymal stem cells. Int. J. Biol. Macromol. 2018;112:448–460. doi: 10.1016/j.ijbiomac.2018.01.181. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

