Systemic Dermatitis Model Mice Exhibit Atrophy of Visceral Adipose Tissue and Increase Stromal Cells via Skin-Derived Inflammatory Cytokines
- PMID: 32397568
- PMCID: PMC7247662
- DOI: 10.3390/ijms21093367
Systemic Dermatitis Model Mice Exhibit Atrophy of Visceral Adipose Tissue and Increase Stromal Cells via Skin-Derived Inflammatory Cytokines
Abstract
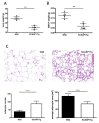
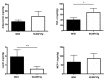
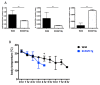
: Adipose tissue (AT) is the largest endocrine organ, producing bioactive products called adipocytokines, which regulate several metabolic pathways, especially in inflammatory conditions. On the other hand, there is evidence that chronic inflammatory skin disease is closely associated with vascular sclerotic changes, cardiomegaly, and severe systemic amyloidosis in multiple organs. In psoriasis, a common chronic intractable inflammatory skin disease, several studies have shown that adipokine levels are associated with disease severity. Chronic skin disease is also associated with metabolic syndrome, including abnormal tissue remodeling; however, the mechanism is still unclear. We addressed this problem using keratin 14-specific caspase-1 overexpressing transgenic (KCASP1Tg) mice with severe erosive dermatitis from 8 weeks of age, followed by re-epithelization. The whole body and gonadal white AT (GWAT) weights were decreased. Each adipocyte was large in number, small in size and irregularly shaped; abundant inflammatory cells, including activated CD4+ or CD8+ T cells and toll-like receptor 4/CD11b-positive activated monocytes, infiltrated into the GWAT. We assumed that inflammatory cytokine production in skin lesions was the key factor for this lymphocyte/monocyte activation and AT dysregulation. We tested our hypothesis that the AT in a mouse dermatitis model shows an impaired thermogenesis ability due to systemic inflammation. After exposure to 4 °C, the mRNA expression of the thermogenic gene uncoupling protein 1 in adipocytes was elevated; however, the body temperature of the KCASP1Tg mice decreased rapidly, revealing an impaired thermogenesis ability of the AT due to atrophy. Tumor necrosis factor (TNF)-α, IL-1β and interferon (INF)-γ levels were significantly increased in KCASP1Tg mouse ear skin lesions. To investigate the direct effects of these cytokines, BL/6 wild mice were administered intraperitoneal TNF-α, IL-1β and INF-γ injections, which resulted in small adipocytes with abundant stromal cell infiltration, suggesting those cytokines have a synergistic effect on adipocytes. The systemic dermatitis model mice showed atrophy of AT and increased stromal cells. These findings were reproducible by the intraperitoneal administration of inflammatory cytokines whose production was increased in inflamed skin lesions.
Keywords: adiponectin; adipose tissue; cytokine; leptin; skin inflammation; thermogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

