Identification of bioactive molecules from tea plant as SARS-CoV-2 main protease inhibitors
- PMID: 32397940
- PMCID: PMC7256349
- DOI: 10.1080/07391102.2020.1766572
Identification of bioactive molecules from tea plant as SARS-CoV-2 main protease inhibitors
Abstract
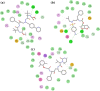
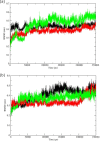
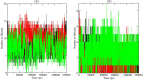
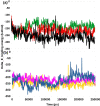
The SARS-CoV-2 is the causative agent of COVID-19 pandemic that is causing a global health emergency. The lack of targeted therapeutics and limited treatment options have triggered the scientific community to develop new vaccines or small molecule therapeutics against various targets of SARS-CoV-2. The main protease (Mpro) is a well characterized and attractive drug target because of its crucial role in processing of the polyproteins which are required for viral replication. In order to provide potential lead molecules against the Mpro for clinical use, we docked a set of 65 bioactive molecules of Tea plant followed by exploration of the vast conformational space of protein-ligand complexes by long term molecular dynamics (MD) simulations (1.50 µs). Top three bioactive molecules (Oolonghomobisflavan-A, Theasinensin-D, and Theaflavin-3-O-gallate) were selected by comparing their docking scores with repurposed drugs (Atazanavir, Darunavir, and Lopinavir) against SARS-CoV-2. Oolonghomobisflavan-A molecule showed a good number of hydrogen bonds with Mpro and higher MM-PBSA binding energy when compared to all three repurposed drug molecules. during the time of simulation. This study showed Oolonghomobisflavan-A as a potential bioactive molecule to act as an inhibitor for the Mpro of SARS-CoV-2.
Keywords: COVID-19; SARS-CoV-2; bioactive molecules; main protease.
Figures

References
-
- Abraham M. J., Murtola T., Schulz R., Páll S., Smith J. C., Hess B., & Lindahl E. (2015). GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX, 1-2, 19–25. 10.1016/j.softx.2015.06.001 - DOI
-
- Atazanavir | C38H52N6O7 - PubChem (n.d.). Retrieved April 18, 2020, from https://pubchem.ncbi.nlm.nih.gov/compound/Atazanavir
-
- Berendsen H. J. C., Postma J. P. M., van Gunsteren W. F., DiNola A., & Haak J. R. (1984). Molecular dynamics with coupling to an external bath. The Journal of Chemical Physics, 81(8), 3684–3690. 10.1063/1.448118 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
