PEACE V - Salvage Treatment of OligoRecurrent nodal prostate cancer Metastases (STORM): a study protocol for a randomized controlled phase II trial
- PMID: 32398040
- PMCID: PMC7216526
- DOI: 10.1186/s12885-020-06911-4
PEACE V - Salvage Treatment of OligoRecurrent nodal prostate cancer Metastases (STORM): a study protocol for a randomized controlled phase II trial
Abstract
Background: Pelvic nodal recurrences are being increasingly diagnosed with the introduction of new molecular imaging techniques, like choline and PSMA PET-CT, in the restaging of recurrent prostate cancer (PCa). At this moment, there are no specific treatment recommendations for patients with limited nodal recurrences and different locoregional treatment approaches are currently being used, mostly by means of metastasis-directed therapies (MDT): salvage lymph node dissection (sLND) or stereotactic body radiotherapy (SBRT). Since the majority of patients treated with MDT relapse within 2 years in adjacent lymph node regions, with an estimated median time to progression of 12-18 months, combining MDT with whole pelvic radiotherapy (WPRT) may improve oncological outcomes in these patients. The aim of this prospective multicentre randomized controlled phase II trial is to assess the impact of the addition of WPRT to MDT and short-term androgen deprivation therapy (ADT) on metastasis-free survival (MFS) in the setting of oligorecurrent pelvic nodal recurrence.
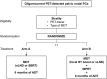
Methods & design: Patients diagnosed with PET-detected pelvic nodal oligorecurrence (≤5 nodes) following radical local treatment for PCa, will be randomized in a 1:1 ratio between arm A: MDT and 6 months of ADT, or arm B: WPRT added to MDT and 6 months of ADT. Patients will be stratified by type of PET-tracer (choline, FACBC or PSMA) and by type of MDT (sLND or SBRT). The primary endpoint is MFS and the secondary endpoints include clinical and biochemical progression-free survival (PFS), prostate cancer specific survival, quality of life (QoL), toxicity and time to castration-resistant prostate cancer (CRPC) and to palliative ADT. Estimated study completion: December 31, 2023.
Discussion: This is the first prospective multicentre randomized phase II trial assessing the potential of combined WPRT and MDT as compared to MDT alone on MFS for patients with nodal oligorecurrent PCa.
Trial registration: ClinicalTrials.gov Identifier: NCT03569241, registered June 14, 2018, ; Identifier on Swiss National Clinical Trials Portal (SNCTP): SNCTP000002947, registered June 14, 2018.
Keywords: Androgen deprivation therapy; Metastasis-directed therapy; Oligometastases; Oligorecurrence; Prostate cancer; Quality of life; Salvage lymph node dissection; Stereotactic body radiotherapy; Survival; Whole pelvic radiotherapy.
Conflict of interest statement
PO:
Research funding: Merck (Inst), Varian (Inst)
Consulting or Advisory Role: Ferring Pharmaceuticals (Inst), Bayer AG (Inst), Janssen (Inst)
Travel, Accommodations, Expenses: Ipsen, Ferring Pharmaceuticals
AZ:
Research funding: Janssen, AstraZeneca
Advisory board: IPSEN
Speaker fees: Astellas, Janssen.
The other authors declare that they have no competing interests for this trial.
Figures
References
-
- Mottet N, Briers E, Cornford P, De Santis M, Fanti S, Gillessen S, Grummet J, Henry AM, Lam TB, Mason MD, van der Kwast TH, van der Poel HG, Rouvière O, Tilki D, Wiegel T, Fossati N, Gross T, Lardas M, Liew M, Moris L, Schoots IG, Willemse P-PM. Guidelines Associates. In: Van den Broeck MC T, editor. EAU - EANM - ESTRO - ESUR - SIOG Guidelines on Prostate Cancer 2019 EAU. 2019.
-
- Graziani T, Ceci F, Castellucci P, Polverari G, Lima GM, Lodi F, et al. (11) C-choline PET/CT for restaging prostate cancer. Results from 4,426 scans in a single-Centre patient series. Eur J Nucl Med Mol Imaging. 2016;43(11):1971–1979. - PubMed
-
- Perera M, Papa N, Christidis D, Wetherell D, Hofman MS, Murphy DG, et al. Sensitivity, specificity, and predictors of positive (68)Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate Cancer: a systematic review and meta-analysis. Eur Urol. 2016;70(6):926–937. - PubMed
-
- Ost P, Bossi A, Decaestecker K, De Meerleer G, Giannarini G, Karnes RJ, et al. Metastasis-directed therapy of regional and distant recurrences after curative treatment of prostate cancer: a systematic review of the literature. Eur Urol. 2015;67(5):852–863. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous