Access to routinely collected health data for clinical trials - review of successful data requests to UK registries
- PMID: 32398093
- PMCID: PMC7218527
- DOI: 10.1186/s13063-020-04329-8
Access to routinely collected health data for clinical trials - review of successful data requests to UK registries
Abstract
Background: Clinical trials generally each collect their own data despite routinely collected health data (RCHD) increasing in quality and breadth. Our aim is to quantify UK-based randomised controlled trials (RCTs) accessing RCHD for participant data, characterise how these data are used and thereby recommend how more trials could use RCHD.
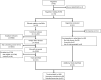
Methods: We conducted a systematic review of RCTs accessing RCHD from at least one registry in the UK between 2013 and 2018 for the purposes of informing or supplementing participant data. A list of all registries holding RCHD in the UK was compiled. In cases where registries published release registers, these were searched for RCTs accessing RCHD. Where no release register was available, registries were contacted to request a list of RCTs. For each identified RCT, information was collected from all publicly available sources (release registers, websites, protocol etc.). The search and data extraction were undertaken between January and May 2019.
Results: We identified 160 RCTs accessing RCHD between 2013 and 2018 from a total of 22 registries; this corresponds to only a very small proportion of all UK RCTs (about 3%). RCTs accessing RCHD were generally large (median sample size 1590), commonly evaluating treatments for cancer or cardiovascular disease. Most of the included RCTs accessed RCHD from NHS Digital (68%), and the most frequently accessed datasets were mortality (76%) and hospital visits (55%). RCHD was used to inform the primary trial (82%) and long-term follow-up (57%). There was substantial variation in how RCTs used RCHD to inform participant outcome measures. A limitation was the lack of information and transparency from registries and RCTs with respect to which datasets have been accessed and for what purposes.
Conclusions: In the last five years, only a small minority of UK-based RCTs have accessed RCHD to inform participant data. We ask for improved accessibility, confirmed data quality and joined-up thinking between the registries and the regulatory authorities.
Trial registration: PROSPERO CRD42019123088.
Keywords: RCT; Registry; Routinely collected health data; Systematic review.
Conflict of interest statement
All authors have completed the International Committee of Medical Journal Editors uniform disclosure form at
Figures
References
-
- Raftery J, Young A, Stanton L, Milne R, Cook A, Turner D, et al. Clinical trial metadata: defining and extracting metadata on the design, conduct, results and costs of 125 randomised clinical trials funded by the National Institute for Health Research Health Technology Assessment programme. Health Technol Assess (Winchester, England) 2015;19(11):1–138. doi: 10.3310/hta19110. - DOI - PMC - PubMed
-
- https://www.hdruk.ac.uk/research/research-priorities/21st-century-clinic... (accessed 10 Mar 2020).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

