Promoter methylation changes in ALOX12 and AIRE1: novel epigenetic markers for atherosclerosis
- PMID: 32398127
- PMCID: PMC7218560
- DOI: 10.1186/s13148-020-00846-0
Promoter methylation changes in ALOX12 and AIRE1: novel epigenetic markers for atherosclerosis
Abstract
Background: Atherosclerosis is the main cause of cardiovascular diseases such as ischemic stroke and coronary heart disease. Gene-specific promoter methylation changes have been suggested as one of the causes underlying the development of atherosclerosis. We aimed to identify and validate specific genes that are differentially expressed through promoter methylation in atherosclerotic plaques. We performed the present study in four steps: (1) profiling and identification of gene-specific promoter methylation changes in atherosclerotic tissues; (2) validation of the promoter methylation changes of genes in plaques by comparison with non-plaque intima; (3) evaluation of promoter methylation status of the genes in vascular cellular components composing atherosclerotic plaques; and (4) evaluation of promoter methylation differences in genes among monocytes, T cells, and B cells isolated from the blood of ischemic stroke patients.
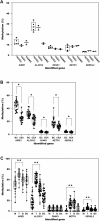
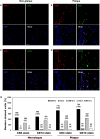
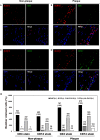
Results: Upon profiling, AIRE1, ALOX12, FANK1, NETO1, and SERHL2 were found to have displayed changes in promoter methylation. Of these, AIRE1 and ALOX12 displayed higher methylation levels in plaques than in non-plaque intima, but lower than those in the buffy coat of blood. Between inflammatory cells, the three genes were significantly less methylated in monocytes than in T and B cells. In the vascular cells, AIRE1 methylation was lower in endothelial and smooth muscle cells. ALOX12 methylation was higher in endothelial, but lower in smooth muscle cells. Immunofluorescence staining showed that co-localization of ALOX12 and AIRE1 was more frequent in CD14(+)-monocytes than in CD4(+)-T cell in plaque than in non-plaque intima.
Conclusions: Promoter methylation changes in AIRE1 and ALOX12 occur in atherosclerosis and can be considered as novel epigenetic markers.
Keywords: Atherosclerosis; epigenetics; markers; promoter methylation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Mendis S, Puska P, Norrving B. Chapter 3. The underlying pathology of heart attacks and strokes. In: Global Atlas on Cardiovascular Disease Prevention and Control. World Health Organization in collaboration with the World Heart Federation and the World Stroke Organization. 2011: 3-18.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

