Behavioral and electrophysiological evidence for a neuroprotective role of aquaporin-4 in the 5xFAD transgenic mice model
- PMID: 32398151
- PMCID: PMC7218576
- DOI: 10.1186/s40478-020-00936-3
Behavioral and electrophysiological evidence for a neuroprotective role of aquaporin-4 in the 5xFAD transgenic mice model
Abstract
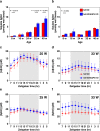
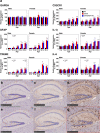
Aquaporin-4 (AQP4) has been suggested to be involved in the pathogenesis of neurodegenerative diseases including Alzheimer's disease (AD), which may be due to the modulation of neuroinflammation or the impairment of interstitial fluid bulk flow system in the central nervous system. Here, we show an age-dependent impairment of several behavioral outcomes in 5xFAD AQP4 null mice. Twenty-four-hour video recordings and computational analyses of their movement revealed that the nighttime motion of AQP4-deficient 5xFAD mice was progressively reduced between 20 and 36 weeks of age, with a sharp deterioration occurring between 30 and 32 weeks. This reduction in nighttime motion was accompanied by motor dysfunction and epileptiform neuronal activities, demonstrated by increased abnormal spikes by electroencephalography. In addition, all AQP4-deficient 5xFAD mice exhibited convulsions at least once during the period of the analysis. Interestingly, despite such obvious phenotypes, parenchymal amyloid β (Aβ) deposition, reactive astrocytosis, and activated microgliosis surrounding amyloid plaques were unchanged in the AQP4-deficient 5xFAD mice relative to 5xFAD mice. Taken together, our data indicate that AQP4 deficiency greatly accelerates an age-dependent deterioration of neuronal function in 5xFAD mice associated with epileptiform neuronal activity without significantly altering Aβ deposition or neuroinflammation in this mouse model. We therefore propose that there exists another pathophysiological phase in AD which follows amyloid plaque deposition and neuroinflammation and is sensitive to AQP4 deficiency.
Keywords: 5xFAD; Alzheimer’s disease; Amyloid β; Aquaporin-4; Epilepsy.
Conflict of interest statement
Masato Yasui has received funding for the research from Suntory Global Innovation Center Ltd.
The other authors declare that they have no competing interests.
Figures




References
-
- Born HA. Seizures in Alzheimer's disease. Neuroscience. 2015;286:251–263. - PubMed
-
- Carare RO, Bernardes-Silva M, Newman TA, Page AM, Nicoll JA, Perry VH, et al. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol Appl Neurobiol. 2008;34:131–144. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

