The Post-COVID-19 Functional Status scale: a tool to measure functional status over time after COVID-19
- PMID: 32398306
- PMCID: PMC7236834
- DOI: 10.1183/13993003.01494-2020
The Post-COVID-19 Functional Status scale: a tool to measure functional status over time after COVID-19
Abstract
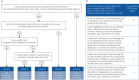
An ordinal tool is proposed to measure the full spectrum of functional outcomes following COVID-19. This “Post-COVID-19 Functional Status (PCFS) scale” can be used for tracking functional status over time as well as for research purposes.
Conflict of interest statement
Conflict of interest: F.A. Klok reports grants from Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Daiichi Sankyo, MSD, Actelion, the Netherlands Thrombosis Foundation and the Dutch Heart Foundation, outside the submitted work. Conflict of interest: G.J.A.M. Boon has nothing to disclose. Conflict of interest: S. Barco has nothing to disclose. Conflict of interest: M. Endres reports grants from DFG under Germany's Excellence Strategy (EXC-2049; 390688087) and Bayer, and personal fees from Bayer, Boehringer Ingelheim, BMS, Daiichi Sankyo, Amgen, GSK, Sanofi, Covidien, Novartis and Pfizer, outside the submitted work. Conflict of interest: J.J.M. Geelhoed has nothing to disclose. Conflict of interest: S. Knauss has nothing to disclose. Conflict of interest: S.A. Rezek has nothing to disclose. Conflict of interest: M.A. Spruit reports grants from the Netherlands Lung Foundation and Stichting Astma Bestrijding, and grants and personal fees from AstraZeneca and Boehringer Ingelheim, outside the submitted work. Conflict of interest: J. Vehreschild has nothing to disclose. Conflict of interest: B. Siegerink has nothing to disclose.
Figures
References
-
- Johns Hopkins University Coronavirus Resource Center. https://coronavirus.jhu.edu/ Date last accessed: April 2020.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
