Intravitreal ranibizumab and dexamethasone implant injections as primary treatment of diabetic macular edema: simultaneously double protocol
- PMID: 32398852
- PMCID: PMC8027799
- DOI: 10.1038/s41433-020-0949-2
Intravitreal ranibizumab and dexamethasone implant injections as primary treatment of diabetic macular edema: simultaneously double protocol
Abstract
Purpose: To assess the 12-month efficacy and safety of simultaneously administered intravitreal dexamethasone implant (DEX implant) and ranibizumab (simultaneous double protocol) injections in comparison with ranibizumab monotherapy as the first-line treatment of diabetic macular oedema (DMO).
Methods: Prospective, consecutive, clinical interventional study. Patients were randomized into two groups: 24 naive DMO patients (34 eyes) who received simultaneous double-protocol therapy and 22 DMO patients (34 eyes) who received ranibizumab monotherapy were included. Monthly ranibizumab (0.5 mg) was administered for the first 6 months and later on, an as-needed treatment basis. DEX implant injection was performed at any time during the loading dose of the three consecutive monthly injections of ranibizumab, and with as-needed reinjections of ranibizumab from 6th month onwards. Change in visual acuity was the primary efficacy endpoint. Secondary efficacy endpoints were a gain of ≥15 letters and a change in the central foveal thickness.
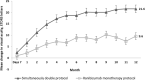
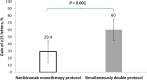
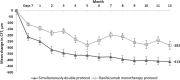
Results: Mean BCVA increased from baseline to month 12 in the simultaneously double-protocol therapy group compared with the ranibizumab monotherapy group (21.6 versus 9.6 letters [P < 0.001]). The corresponding proportions of eyes gaining ≥15 letters were 60% versus 29.4% (P < 0.0001). Moreover, the mean reductions in the central foveal thickness were 413 versus 282 µm (P = 0.001). At 12 month, the simultaneous double-protocol therapy decreased a significant number of foveal cysts and subfoveal neuroretinal detachment compared with those by ranibizumab monotherapy.
Conclusions: The simultaneous addition of DEX implant at any time during the three monthly loading doses of ranibizumab in patients with DMO significantly improved the visual outcomes and revealed superior anatomic outcomes than those with the ranibizumab monotherapy.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Klein R, Klein B. Vision disorders in diabetes. In: National Diabetes Data Group, editor. Diabetes in America. 2nd ed. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 1995. p. 293–338.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

