A serological assay to detect SARS-CoV-2 seroconversion in humans
- PMID: 32398876
- PMCID: PMC8183627
- DOI: 10.1038/s41591-020-0913-5
A serological assay to detect SARS-CoV-2 seroconversion in humans
Abstract
Here, we describe a serological enzyme-linked immunosorbent assay for the screening and identification of human SARS-CoV-2 seroconverters. This assay does not require the handling of infectious virus, can be adjusted to detect different antibody types in serum and plasma and is amenable to scaling. Serological assays are of critical importance to help define previous exposure to SARS-CoV-2 in populations, identify highly reactive human donors for convalescent plasma therapy and investigate correlates of protection.
Conflict of interest statement
Competing interests
Mount Sinai is in the process of licensing out assays to commercial entities based on the assays described here and has filed for patent protection.
Figures


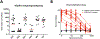
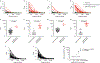

Update of
-
A serological assay to detect SARS-CoV-2 seroconversion in humans.medRxiv [Preprint]. 2020 Apr 16:2020.03.17.20037713. doi: 10.1101/2020.03.17.20037713. medRxiv. 2020. Update in: Nat Med. 2020 Jul;26(7):1033-1036. doi: 10.1038/s41591-020-0913-5. PMID: 32511441 Free PMC article. Updated. Preprint.
Comment in
-
The serostatus approach to fighting COVID-19.Int J Infect Dis. 2020 May;94:53-54. doi: 10.1016/j.ijid.2020.03.080. Epub 2020 Apr 7. Int J Infect Dis. 2020. PMID: 32276044 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

