Exercise-Induced Adaptations to the Mouse Striatal Adenosine System
- PMID: 32399024
- PMCID: PMC7204111
- DOI: 10.1155/2020/5859098
Exercise-Induced Adaptations to the Mouse Striatal Adenosine System
Abstract
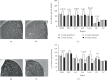
Adenosine acts as a key regulator of striatum activity, in part, through the antagonistic modulation of dopamine activity. Exercise can increase adenosine activity in the brain, which may impair dopaminergic functions in the striatum. Therefore, long-term repeated bouts of exercise may subsequently generate plasticity in striatal adenosine systems in a manner that promotes dopaminergic activity. This study investigated the effects of long-term voluntary wheel running on adenosine 1 (A1R), adenosine 2A (A2AR), dopamine 1 (D1R), and dopamine 2 (D2R) receptor protein expression in adult mouse dorsal and ventral striatum structures using immunohistochemistry. In addition, equilibrative nucleoside transporter 1 (ENT1) protein expression was examined after wheel running, as ENT1 regulates the bidirectional flux of adenosine between intra- and extracellular space. The results suggest that eight weeks of running wheel access spared age-related increases of A1R and A2AR protein concentrations across the dorsal and ventral striatal structures. Wheel running mildly reduced ENT1 protein levels in ventral striatum subregions. Moreover, wheel running mildly increased D2R protein density within striatal subregions in the dorsal medial striatum, nucleus accumbens core, and the nucleus accumbens shell. However, D1R protein expression in the striatum was unchanged by wheel running. These data suggest that exercise promotes adaptations to striatal adenosine systems. Exercise-reduced A1R and A2AR and exercise-increased D2R protein levels may contribute to improved dopaminergic signaling in the striatum. These findings may have implications for cognitive and behavioral processes, as well as motor and psychiatric diseases that involve the striatum.
Copyright © 2020 Ella E. Bauer et al.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this article.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

