New developments in chondrocyte ER stress and related diseases
- PMID: 32399188
- PMCID: PMC7194456
- DOI: 10.12688/f1000research.22275.1
New developments in chondrocyte ER stress and related diseases
Abstract
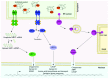
Cartilage comprises a single cell type, the chondrocyte, embedded in a highly complex extracellular matrix. Disruption to the cartilage growth plate leads to reduced bone growth and results in a clinically diverse group of conditions known as genetic skeletal diseases (GSDs). Similarly, long-term degradation of articular cartilage can lead to osteoarthritis (OA), a disease characterised by joint pain and stiffness. As professionally secreting cells, chondrocytes are particularly susceptible to endoplasmic reticulum (ER) stress and this has been identified as a core disease mechanism in a group of clinically and pathologically related GSDs. If unresolved, ER stress can lead to chondrocyte cell death. Recent interest has focused on ER stress as a druggable target for GSDs and this has led to the first clinical trial for a GSD by repurposing an antiepileptic drug. Interestingly, ER stress markers have also been associated with OA in multiple cell and animal models and there is increasing interest in it as a possible therapeutic target for treatment. In summary, chondrocyte ER stress has been identified as a core disease mechanism in GSDs and as a contributory factor in OA. Thus, chondrocyte ER stress is a unifying factor for both common and rare cartilage-related diseases and holds promise as a novel therapeutic target.
Keywords: cartilage; chondrocyte; drug repurposing; endoplasmic reticulum; osteoarthritis; skeletal dysplasia.
Copyright: © 2020 Briggs MD et al.
Conflict of interest statement
No competing interests were disclosed.No competing interests were disclosed.No competing interests were disclosed.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

